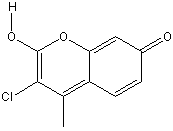
3-chloro-7-hydroxy-4-methylcoumarin
Coumaphos metabolite. Umbelliferone 7-hydroxycoumarins
General
Type : Coumarin
Chemical_Nomenclature : 3-chloro-7-Hydroxy-4-methylcoumarin
Canonical SMILES : CC1=C(C(=O)OC2=C1C=CC(=C2)O)Cl
InChI : InChI=1S\/C10H7ClO3\/c1-5-7-3-2-6(12)4-8(7)14-10(13)9(5)11\/h2-4,12H,1H3
InChIKey : ODZHLDRQCZXQFQ-UHFFFAOYSA-N
Other name(s) : Chlorferone,Chlorferron,3-Chloro-4-methyl-7-hydroxycoumarin,7-Hydroxy-4-methyl-3-chlorocoumarin,Coumarin, 3-chloro-7-hydroxy-4-methyl,CHMC
MW : 210.6
Formula : C10H7ClO3
CAS_number : 6174-86-3
PubChem : 5355079
UniChem : ODZHLDRQCZXQFQ-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 3-chloro-7-hydroxy-4-methylcoumarin ligand of proteins in family: BCHE || ACHE
Stucture :
Protein :
References (2)
| Title : Reversible inhibition of acetylcholinesterase and butyrylcholinesterase by 4,4'-bipyridine and by a coumarin derivative - Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_119 |
| Author(s) : Simeon-Rudolf V , Kovarik Z , Radic Z , Reiner E |
| Ref : Chemico-Biological Interactions , 119-120 :119 , 1999 |
| Abstract : Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_119 |
| ESTHER : Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_119 |
| PubMedSearch : Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_119 |
| PubMedID: 10421445 |
| Title : Biotransformation and disposition of the coumaphos metabolite 3-chloro-4-methyl-(4-14C)-7-hydroxycoumarin in rats - Malik_1981_Arch.Toxicol_48_51 |
| Author(s) : Malik JK , Lay JP , Klein W , Korte F |
| Ref : Archives of Toxicology , 48 :51 , 1981 |
| Abstract : Malik_1981_Arch.Toxicol_48_51 |
| ESTHER : Malik_1981_Arch.Toxicol_48_51 |
| PubMedSearch : Malik_1981_Arch.Toxicol_48_51 |
| PubMedID: 7283748 |