5NN0-cpd3
The compound has a picomolar inhibition constant versus BChE, inhibits BChE ex vivo, and is non-cytotoxic. It crosses the blood-brain barrier, and improves memory, cognitive functions, and learning abilities of mice in a scopolamine model of dementia
General
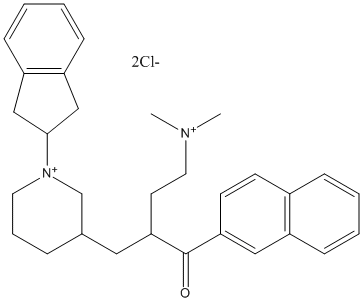
Type : Piperidine,Inden
Chemical_Nomenclature : (+\/-)-N-((1-(2,3-Dihydro-1H-inden-2-yl)piperidin-3-yl)methyl)-N-(2-(dimethylamino)ethyl)-2-naphthamide
Canonical SMILES : CN(C)CCN(C[C@@H]1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1
InChI : InChI=1S\/C30H37N3O\/c1-31(2)16-17-33(30(34)28-14-13-24-9-3-4-10-25(24)18-28)22-23-8-7-15-32(21-23)29-19-26-11-5-6-12-27(26)20-29\/h3-6,9-14,18,23,29H,7-8,15-17,19-22H2,1-2H3\/t23-\/m1\/s1
InChIKey : OFDHKZMZEXORRX-HSZRJFAPSA-N
Other name(s) : 92H
MW : 525.55
Formula : C31H38Cl2N2O
CAS_number :
PubChem : 132471735
UniChem : OFDHKZMZEXORRX-HSZRJFAPSA-N
IUPHAR :
Wikipedia :
Target
Families : 5NN0-cpd3 ligand of proteins in family: BCHE
Stucture : 5NN0 Human butyrylcholinesterase in complex with inhibitor with picomolar activity
Protein : human-BCHE
References (1)
| Title : The Magic of Crystal Structure-Based Inhibitor Optimization: Development of a Butyrylcholinesterase Inhibitor with Picomolar Affinity and in Vivo Activity - Kosak_2018_J.Med.Chem_61_119 |
| Author(s) : Kosak U , Brus B , Knez D , Zakelj S , Trontelj J , Pislar A , Sink R , Jukic M , Zivin M , Podkowa A , Nachon F , Brazzolotto X , Stojan J , Kos J , Coquelle N , Salat K , Colletier JP , Gobec S |
| Ref : Journal of Medicinal Chemistry , 61 :119 , 2018 |
| Abstract : Kosak_2018_J.Med.Chem_61_119 |
| ESTHER : Kosak_2018_J.Med.Chem_61_119 |
| PubMedSearch : Kosak_2018_J.Med.Chem_61_119 |
| PubMedID: 29227101 |
| Gene_locus related to this paper: human-BCHE |