Harmine
Alkaloid isolated from seeds of Peganum harmala; Zygophyllacea. It is identical to banisterine, or telepathine, from Banisteria caapi and is one of the active ingredients of hallucinogenic drinks made in the western Amazon region from related plants. It has a role as a metabolite, an anti-HIV agent and an EC 1.4.3.4 (monoamine oxidase) inhibitor.
General
Type : Natural,Alkaloid,Indole,beta-carboline,Not A\/B H target
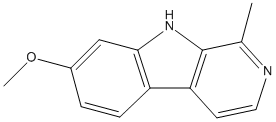
Chemical_Nomenclature : 7-methoxy-1-methyl-9H-pyrido[3,4-b]indole
Canonical SMILES : CC1=NC=CC2=C1NC3=C2C=CC(=C3)OC
InChI : InChI=1S\/C13H12N2O\/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13\/h3-7,15H,1-2H3
InChIKey : BXNJHAXVSOCGBA-UHFFFAOYSA-N
Other name(s) : Banisterine,Telepathine
MW : 212.25
Formula : C13H12N2O
CAS_number : 442-51-3
PubChem : 5280953
UniChem : BXNJHAXVSOCGBA-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families :
Stucture :
Protein :
References (11)
| Title : A preliminary study on the neurotoxic mechanism of harmine in Caenorhabditis elegans - Sun_2021_Comp.Biochem.Physiol.C.Toxicol.Pharmacol__109038 |
| Author(s) : Sun Q , Liu C , Jiang K , Fang Y , Kong C , Fu J , Liu Y |
| Ref : Comparative Biochemistry & Physiology C Toxicol Pharmacol , :109038 , 2021 |
| Abstract : Sun_2021_Comp.Biochem.Physiol.C.Toxicol.Pharmacol__109038 |
| ESTHER : Sun_2021_Comp.Biochem.Physiol.C.Toxicol.Pharmacol__109038 |
| PubMedSearch : Sun_2021_Comp.Biochem.Physiol.C.Toxicol.Pharmacol__109038 |
| PubMedID: 33794375 |
| Title : Effect of Harmine and Its Derivatives Against Echinococcus granulosus and Comparison of DNA Damage Targets - Gong_2020_J.Biomed.Nanotechnol_16_827 |
| Author(s) : Gong Y , Lv S , Tian C , Gao Y , Chen B , Wen L , Gao H , Aimaiti W , Ma R , Zhao J , Wang J |
| Ref : J Biomed Nanotechnol , 16 :827 , 2020 |
| Abstract : Gong_2020_J.Biomed.Nanotechnol_16_827 |
| ESTHER : Gong_2020_J.Biomed.Nanotechnol_16_827 |
| PubMedSearch : Gong_2020_J.Biomed.Nanotechnol_16_827 |
| PubMedID: 33187579 |
| Title : Pharmacological effects of harmine and its derivatives: a review - Zhang_2020_Arch.Pharm.Res_43_1259 |
| Author(s) : Zhang L , Li D , Yu S |
| Ref : Arch Pharm Res , 43 :1259 , 2020 |
| Abstract : Zhang_2020_Arch.Pharm.Res_43_1259 |
| ESTHER : Zhang_2020_Arch.Pharm.Res_43_1259 |
| PubMedSearch : Zhang_2020_Arch.Pharm.Res_43_1259 |
| PubMedID: 33206346 |
| Title : Mechanism-based pharmacokinetics-pharmacodynamics studies of harmine and harmaline on neurotransmitters regulatory effects in healthy rats: Challenge on monoamine oxidase and acetylcholinesterase inhibition - Jiang_2019_Phytomedicine_62_152967 |
| Author(s) : Jiang B , Meng L , Zou N , Wang H , Li S , Huang L , Cheng X , Wang Z , Chen W , Wang C |
| Ref : Phytomedicine , 62 :152967 , 2019 |
| Abstract : Jiang_2019_Phytomedicine_62_152967 |
| ESTHER : Jiang_2019_Phytomedicine_62_152967 |
| PubMedSearch : Jiang_2019_Phytomedicine_62_152967 |
| PubMedID: 31154274 |
| Title : Potential Pharmacokinetic Drug(-)Drug Interaction Between Harmine, a Cholinesterase Inhibitor, and Memantine, a Non-Competitive N-Methyl-d-Aspartate Receptor Antagonist - Zhang_2019_Molecules_24_ |
| Author(s) : Zhang Y , Li S , Wang Y , Deng G , Cao N , Wu C , Ding W , Cheng X , Wang C |
| Ref : Molecules , 24 : , 2019 |
| Abstract : Zhang_2019_Molecules_24_ |
| ESTHER : Zhang_2019_Molecules_24_ |
| PubMedSearch : Zhang_2019_Molecules_24_ |
| PubMedID: 30978991 |
| Title : Analogous beta-Carboline Alkaloids Harmaline and Harmine Ameliorate Scopolamine-Induced Cognition Dysfunction by Attenuating Acetylcholinesterase Activity, Oxidative Stress, and Inflammation in Mice - Li_2018_Front.Pharmacol_9_346 |
| Author(s) : Li SP , Wang YW , Qi SL , Zhang YP , Deng G , Ding WZ , Ma C , Lin QY , Guan HD , Liu W , Cheng XM , Wang CH |
| Ref : Front Pharmacol , 9 :346 , 2018 |
| Abstract : Li_2018_Front.Pharmacol_9_346 |
| ESTHER : Li_2018_Front.Pharmacol_9_346 |
| PubMedSearch : Li_2018_Front.Pharmacol_9_346 |
| PubMedID: 29755345 |
| Title : Effects of the Natural beta-Carboline Alkaloid Harmine, a Main Constituent of Ayahuasca, in Memory and in the Hippocampus: A Systematic Literature Review of Preclinical Studies - Dos_2016_J.Psychoactive.Drugs__1 |
| Author(s) : Dos Santos RG , Hallak JE |
| Ref : J Psychoactive Drugs , :1 , 2016 |
| Abstract : Dos_2016_J.Psychoactive.Drugs__1 |
| ESTHER : Dos_2016_J.Psychoactive.Drugs__1 |
| PubMedSearch : Dos_2016_J.Psychoactive.Drugs__1 |
| PubMedID: 27918874 |
| Title : Synthesis, cytotoxic, anti-lipoxygenase and anti-acetylcholinesterase capacities of novel derivatives from harmine - Filali_2016_J.Enzyme.Inhib.Med.Chem_31(sup1)_23 |
| Author(s) : Filali I , Belkacem MA , Ben Nejma A , Souchard JP , Ben Jannet H , Bouajila J |
| Ref : J Enzyme Inhib Med Chem , 31(sup1) :23 , 2016 |
| Abstract : Filali_2016_J.Enzyme.Inhib.Med.Chem_31(sup1)_23 |
| ESTHER : Filali_2016_J.Enzyme.Inhib.Med.Chem_31(sup1)_23 |
| PubMedSearch : Filali_2016_J.Enzyme.Inhib.Med.Chem_31(sup1)_23 |
| PubMedID: 27028352 |
| Title : Synthesis of New Harmine Isoxazoles and Evaluation of their Potential Anti-Alzheimer, Anti-inflammatory, and Anticancer Activities - Filali_2016_Med.Chem_12_184 |
| Author(s) : Filali I , Romdhane A , Znati M , Jannet HB , Bouajila J |
| Ref : Med Chem , 12 :184 , 2016 |
| Abstract : Filali_2016_Med.Chem_12_184 |
| ESTHER : Filali_2016_Med.Chem_12_184 |
| PubMedSearch : Filali_2016_Med.Chem_12_184 |
| PubMedID: 26362768 |
| Title : Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities - Filali_2015_J.Enzyme.Inhib.Med.Chem_30_371 |
| Author(s) : Filali I , Bouajila J , Znati M , Bousejra-El Garah F , Ben Jannet H |
| Ref : J Enzyme Inhib Med Chem , 30 :371 , 2015 |
| Abstract : Filali_2015_J.Enzyme.Inhib.Med.Chem_30_371 |
| ESTHER : Filali_2015_J.Enzyme.Inhib.Med.Chem_30_371 |
| PubMedSearch : Filali_2015_J.Enzyme.Inhib.Med.Chem_30_371 |
| PubMedID: 25068731 |
| Title : Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP\/PS1 transgenic mice and scopolamine-induced memory impairment mice - He_2015_Eur.J.Pharmacol_768_96 |
| Author(s) : He D , Wu H , Wei Y , Liu W , Huang F , Shi H , Zhang B , Wu X , Wang C |
| Ref : European Journal of Pharmacology , 768 :96 , 2015 |
| Abstract : He_2015_Eur.J.Pharmacol_768_96 |
| ESTHER : He_2015_Eur.J.Pharmacol_768_96 |
| PubMedSearch : He_2015_Eur.J.Pharmacol_768_96 |
| PubMedID: 26526348 |