IMP-pNP
sarin analogue. Less toxic but giving the same adduct close to parathion methyl parathion
General
Type : Organophosphate,Surrogate OP,Nerve Agent G-series,pNP,Derivative of Sarin
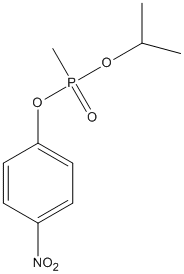
Chemical_Nomenclature : 1-[methyl(propan-2-yloxy)phosphoryl]oxy-4-nitrobenzene
Canonical SMILES : CC(C)OP(=O)(C)OC1=CC=C(C=C1)[N+](=O)[O-]
InChI : InChI=1S\/C10H14NO5P\/c1-8(2)15-17(3,14)16-10-6-4-9(5-7-10)11(12)13\/h4-8H,1-3H3
InChIKey : YSHVIWJFWCEDSQ-UHFFFAOYSA-N
Other name(s) : 4-Nitrophenyl 2-propylmethylphosphonate,BRN 2220854,Isopropyl p-nitrophenyl methylphosphonate,P-Nitrophenyl isopropyl methylphosphonate,4-nitrophenyl propan-2-yl methylphosphonate,ST50983466,NIMP,Isopropyl (4-nitrophenyl) methylphosphonate
MW : 259.2
Formula : C10H14NO5P
CAS_number :
PubChem : 77324
UniChem : YSHVIWJFWCEDSQ-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : IMP-pNP ligand of proteins in family: ACHE || Cholesterol_esterase
Stucture : 6H18 Crystal structure of sarin surrogate NIMP inhibited recombinant human bile salt activated lipase
Protein : human-ACHE || human-CEL
References (10)
| Title : Effects of novel brain-penetrating oxime acetylcholinesterase reactivators on sarin surrogate-induced changes in rat brain gene expression - Dail_2021_J.Biochem.Mol.Toxicol__e22755 |
| Author(s) : Dail ME , Brino MLM , Chambers JE |
| Ref : J Biochem Mol Toxicol , :e22755 , 2021 |
| Abstract : Dail_2021_J.Biochem.Mol.Toxicol__e22755 |
| ESTHER : Dail_2021_J.Biochem.Mol.Toxicol__e22755 |
| PubMedSearch : Dail_2021_J.Biochem.Mol.Toxicol__e22755 |
| PubMedID: 33682265 |
| Title : X-ray structures of human bile-salt activated lipase conjugated to nerve agents surrogates - Touvrey_2019_Toxicology_411_15 |
| Author(s) : Touvrey C , Courageux C , Guillon V , Terreux R , Nachon F , Brazzolotto X |
| Ref : Toxicology , 411 :15 , 2019 |
| Abstract : Touvrey_2019_Toxicology_411_15 |
| ESTHER : Touvrey_2019_Toxicology_411_15 |
| PubMedSearch : Touvrey_2019_Toxicology_411_15 |
| PubMedID: 30359675 |
| Gene_locus related to this paper: human-CEL |
| Title : The inhibition, reactivation and mechanism of VX-, sarin-, fluoro-VX and fluoro-sarin surrogates following their interaction with HuAChE and HuBuChE - Chao_2018_Chem.Biol.Interact_291_220 |
| Author(s) : Chao CK , Balasubramanian N , Gerdes JM , Thompson CM |
| Ref : Chemico-Biological Interactions , 291 :220 , 2018 |
| Abstract : Chao_2018_Chem.Biol.Interact_291_220 |
| ESTHER : Chao_2018_Chem.Biol.Interact_291_220 |
| PubMedSearch : Chao_2018_Chem.Biol.Interact_291_220 |
| PubMedID: 29920286 |
| Title : Comparison of inhibition kinetics of several organophosphates, including some nerve agent surrogates, using human erythrocyte and rat and mouse brain acetylcholinesterase - Coban_2016_Toxicol.Lett_248_39 |
| Author(s) : Coban A , Carr RL , Chambers HW , Willeford KO , Chambers JE |
| Ref : Toxicol Lett , 248 :39 , 2016 |
| Abstract : Coban_2016_Toxicol.Lett_248_39 |
| ESTHER : Coban_2016_Toxicol.Lett_248_39 |
| PubMedSearch : Coban_2016_Toxicol.Lett_248_39 |
| PubMedID: 26965078 |
| Title : Novel brain-penetrating oximes for reactivation of cholinesterase inhibited by sarin and VX surrogates - Chambers_2016_Ann.N.Y.Acad.Sci_1374_52 |
| Author(s) : Chambers JE , Meek EC , Chambers HW |
| Ref : Annals of the New York Academy of Sciences , 1374 :52 , 2016 |
| Abstract : Chambers_2016_Ann.N.Y.Acad.Sci_1374_52 |
| ESTHER : Chambers_2016_Ann.N.Y.Acad.Sci_1374_52 |
| PubMedSearch : Chambers_2016_Ann.N.Y.Acad.Sci_1374_52 |
| PubMedID: 27153507 |
| Title : Testing of novel brain-penetrating oxime reactivators of acetylcholinesterase inhibited by nerve agent surrogates - Chambers_2013_Chem.Biol.Interact_203_135 |
| Author(s) : Chambers JE , Chambers HW , Meek EC , Pringle RB |
| Ref : Chemico-Biological Interactions , 203 :135 , 2013 |
| Abstract : Chambers_2013_Chem.Biol.Interact_203_135 |
| ESTHER : Chambers_2013_Chem.Biol.Interact_203_135 |
| PubMedSearch : Chambers_2013_Chem.Biol.Interact_203_135 |
| PubMedID: 23123249 |
| Title : Synthesis and in vitro and in vivo inhibition potencies of highly relevant nerve agent surrogates - Meek_2012_Toxicol.Sci_126_525 |
| Author(s) : Meek EC , Chambers HW , Coban A , Funck KE , Pringle RB , Ross MK , Chambers JE |
| Ref : Toxicol Sci , 126 :525 , 2012 |
| Abstract : Meek_2012_Toxicol.Sci_126_525 |
| ESTHER : Meek_2012_Toxicol.Sci_126_525 |
| PubMedSearch : Meek_2012_Toxicol.Sci_126_525 |
| PubMedID: 22247004 |
| Title : Asymmetric fluorogenic organophosphates for the development of active organophosphate hydrolases with reversed stereoselectivity - Amitai_2007_Toxicology_233_187 |
| Author(s) : Amitai G , Adani R , Yacov G , Yishay S , Teitlboim S , Tveria L , Limanovich O , Kushnir M , Meshulam H |
| Ref : Toxicology , 233 :187 , 2007 |
| Abstract : Amitai_2007_Toxicology_233_187 |
| ESTHER : Amitai_2007_Toxicology_233_187 |
| PubMedSearch : Amitai_2007_Toxicology_233_187 |
| PubMedID: 17129656 |
| Title : Enhanced stereoselective hydrolysis of toxic organophosphates by directly evolved variants of mammalian serum paraoxonase - Amitai_2006_FEBS.J_273_1906 |
| Author(s) : Amitai G , Gaidukov L , Adani R , Yishay S , Yacov G , Kushnir M , Teitlboim S , Lindenbaum M , Bel P , Khersonsky O , Tawfik DS , Meshulam H |
| Ref : Febs J , 273 :1906 , 2006 |
| Abstract : Amitai_2006_FEBS.J_273_1906 |
| ESTHER : Amitai_2006_FEBS.J_273_1906 |
| PubMedSearch : Amitai_2006_FEBS.J_273_1906 |
| PubMedID: 16640555 |
| Title : New safe method for preparation of sarin-exposed human erythrocytes acetylcholinesterase using non-toxic and stable sarin analogue isopropyl p-nitrophenyl methylphosphonate and its application to evaluation of nerve agent antidotes - Ohta_2006_Pharm.Res_23_2827 |
| Author(s) : Ohta H , Ohmori T , Suzuki S , Ikegaya H , Sakurada K , Takatori T |
| Ref : Pharm Res , 23 :2827 , 2006 |
| Abstract : Ohta_2006_Pharm.Res_23_2827 |
| ESTHER : Ohta_2006_Pharm.Res_23_2827 |
| PubMedSearch : Ohta_2006_Pharm.Res_23_2827 |
| PubMedID: 17096183 |