MMU
General
Type : Natural,Urea derivative
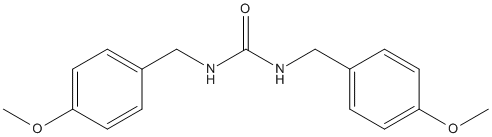
Chemical_Nomenclature : 1,3-bis[(4-methoxyphenyl)methyl]urea
Canonical SMILES : COC1=CC=C(C=C1)CNC(=O)NCC2=CC=C(C=C2)OC
InChI : InChI=1S\/C17H20N2O3\/c1-21-15-7-3-13(4-8-15)11-18-17(20)19-12-14-5-9-16(22-2)10-6-14\/h3-10H,11-12H2,1-2H3,(H2,18,19,20)
InChIKey : PPSSZWOYSHYRJP-UHFFFAOYSA-N
Other name(s) : 1,3-bis(4-methoxybenzyl)urea,1,3-bis[(4-methoxyphenyl)methyl]urea,N,N'-bis(4-methoxybenzyl)urea,ST50667790,NSC108740
MW : 300.35
Formula : C17H20N2O3
CAS_number : 93731-94-3
PubChem : 268493
UniChem : PPSSZWOYSHYRJP-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (3)
| Title : Occurrence of urea-based soluble epoxide hydrolase inhibitors from the plants in the order Brassicales - Kitamura_2017_PLoS.One_12_e0176571 |
| Author(s) : Kitamura S , Morisseau C , Harris TR , Inceoglu B , Hammock BD |
| Ref : PLoS ONE , 12 :e0176571 , 2017 |
| Abstract : Kitamura_2017_PLoS.One_12_e0176571 |
| ESTHER : Kitamura_2017_PLoS.One_12_e0176571 |
| PubMedSearch : Kitamura_2017_PLoS.One_12_e0176571 |
| PubMedID: 28472063 |
| Title : Potent natural soluble epoxide hydrolase inhibitors from Pentadiplandra brazzeana baillon: synthesis, quantification, and measurement of biological activities in vitro and in vivo - Kitamura_2015_PLoS.One_10_e0117438 |
| Author(s) : Kitamura S , Morisseau C , Inceoglu B , Kamita SG , De Nicola GR , Nyegue M , Hammock BD |
| Ref : PLoS ONE , 10 :e0117438 , 2015 |
| Abstract : Kitamura_2015_PLoS.One_10_e0117438 |
| ESTHER : Kitamura_2015_PLoS.One_10_e0117438 |
| PubMedSearch : Kitamura_2015_PLoS.One_10_e0117438 |
| PubMedID: 25659109 |
| Title : Urea derivatives from Pentadiplandra brazzeana - Tsopmo_1999_J.Nat.Prod_62_1435 |
| Author(s) : Tsopmo A , Ngnokam D , Ngamga D , Ayafor JF , Sterner O |
| Ref : Journal of Natural Products , 62 :1435 , 1999 |
| Abstract : Tsopmo_1999_J.Nat.Prod_62_1435 |
| ESTHER : Tsopmo_1999_J.Nat.Prod_62_1435 |
| PubMedSearch : Tsopmo_1999_J.Nat.Prod_62_1435 |
| PubMedID: 10543911 |