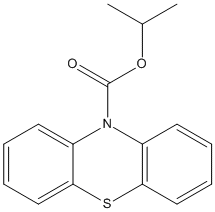
N-10-Phenothiazine-isopropyl-carbamate
(Compound 4 Darvesh et al. 2008) Ki BCHE 18 micro M (for comparison Donepezil 2.21 for BCHE and 0.024 for ACHE), Ka ACHE 1800 M-1min-1 (for comparison rivastigmine 1130 M-1min-1)
General
Type : Phenothiazine,Carbamate,Sulfur Compound
Chemical_Nomenclature : propan-2-yl phenothiazine-10-carboxylate
Canonical SMILES : CC(C)OC(=O)N1C2=CC=CC=C2SC3=CC=CC=C31
InChI : InChI=1S\/C16H15NO2S\/c1-11(2)19-16(18)17-12-7-3-5-9-14(12)20-15-10-6-4-8-13(15)17\/h3-11H,1-2H3
InChIKey : FFXIYTTZCCARMF-UHFFFAOYSA-N
Other name(s) : CHEMBL450026,isopropyl 10H-phenothiazine-10-carboxylate,BDBM50292610
MW : 285.4
Formula : C16H15NO2S
CAS_number :
PubChem : 44568318
UniChem : FFXIYTTZCCARMF-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : N-10-Phenothiazine-isopropyl-carbamate ligand of proteins in family: ACHE || BCHE
Stucture :
Protein : human-ACHE
References (2)
| Title : Carbamates with differential mechanism of inhibition toward acetylcholinesterase and butyrylcholinesterase - Darvesh_2008_J.Med.Chem_51_4200 |
| Author(s) : Darvesh S , Darvesh KV , McDonald RS , Mataija D , Walsh R , Mothana S , Lockridge O , Martin E |
| Ref : Journal of Medicinal Chemistry , 51 :4200 , 2008 |
| Abstract : Darvesh_2008_J.Med.Chem_51_4200 |
| ESTHER : Darvesh_2008_J.Med.Chem_51_4200 |
| PubMedSearch : Darvesh_2008_J.Med.Chem_51_4200 |
| PubMedID: 18570368 |
| Title : Heterocyclic free radicals. Part 8. The influence of the structure and the conformation of the side-chain on the properties of phenothiazine cation-radicals substituted at nitrogen - Clarke_1978_J.Chem.Soc.Perkin.2__1103 |
| Author(s) : Clarke D , Gilbert BC , Hanson P , Kirk CM |
| Ref : J Chem Soc Perkin 2 :1103 , 1978 |
| Abstract : Clarke_1978_J.Chem.Soc.Perkin.2__1103 |
| ESTHER : Clarke_1978_J.Chem.Soc.Perkin.2__1103 |
| PubMedSearch : Clarke_1978_J.Chem.Soc.Perkin.2__1103 |
| PubMedID: |