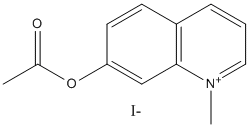
7-acetoxy-N-methylquinolinium
General
Type : Quinoline || Acetate
Chemical_Nomenclature : (1-methylquinolin-1-ium-7-yl) acetate\;iodide
Canonical SMILES : CC(=O)OC1=CC2=C(C=CC=[N+]2C)C=C1.[I-]
InChI : InChI=1S\/C12H12NO2.HI\/c1-9(14)15-11-6-5-10-4-3-7-13(2)12(10)8-11\;\/h3-8H,1-2H3\;1H\/q+1\;\/p-1
InChIKey : IFUAVRXVEJBLFI-UHFFFAOYSA-M
Other name(s) : M7A, 7-Acetoxy-1-methylquinolinium iodide, N-methyl-7-acetoxyquinolinium, 7-Acetoxy-1-methylquinoliniumiodide, AMQI, 7-ACETOXY-1-METHYL-QUINOLINIUM IODID, 7-(acetyloxy)-1-methylquinolin-1-ium iodide
Target
Families : ACHE
References (2)
| Title : Substrate binding to the peripheral site of acetylcholinesterase initiates enzymatic catalysis. Substrate inhibition arises as a secondary effect - Szegletes_1999_Biochemistry_38_122 |
| Author(s) : Szegletes T , Mallender WD , Thomas PJ , Rosenberry TL |
| Ref : Biochemistry , 38 :122 , 1999 |
| Abstract : Szegletes_1999_Biochemistry_38_122 |
| ESTHER : Szegletes_1999_Biochemistry_38_122 |
| PubMedSearch : Szegletes_1999_Biochemistry_38_122 |
| PubMedID: 9890890 |
| Title : Studies of catalysis by acetylcholinesterase. Synergistic effects of inhibitors during the hydrolysis of acetic acid esters - |
| Author(s) : Rosenberry TL , Bernhard SA |
| Ref : Biochemistry , 11 :4308 , 1972 |
| PubMedID: 5079901 |