MGS0274
General
Type : Not A\/B H target || Pro-Drug || Drug
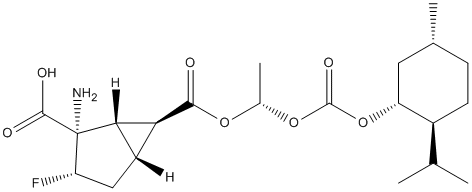
Chemical_Nomenclature : (1S,2S,3S,5R,6S)-2-Amino-3-fluoro-6-(((S)-1-(((((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl)oxy)carbonyl)oxy)ethoxy)carbonyl)bicyclo[3.1.0]hexane-2-carboxylic acid
Canonical SMILES : CC1CCC(C(C1)OC(=O)OC(C)OC(=O)C2C3C2C(C(C3)F)(C(=O)O)N)C(C)C
InChI : InChI=1S\/C21H32FNO7\/c1-9(2)12-6-5-10(3)7-14(12)30-20(27)29-11(4)28-18(24)16-13-8-15(22)21(23,17(13)16)19(25)26\/h9-17H,5-8,23H2,1-4H3,(H,25,26)\/t10-,11+,12+,13+,14-,15+,16+,17+,21+\/m1\/s1
InChIKey : IPAZEPBIOFZFEF-UZEWAWMVSA-N
Other name(s) : SCHEMBL16610461, HY-131336, CS-0133425, TS-134, MGS0274 besylatee
MW : 429.5
Formula : C21H32FNO7
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Carb_B_Chordata
References (3)
| Title : Bottom-up physiologically based pharmacokinetic modeling for predicting the human pharmacokinetic profiles of the ester prodrug MGS0274 and its active metabolite MGS0008, a metabotropic glutamate 2\/3 receptor agonist - Ochi_2022_Xenobiotica__1 |
| Author(s) : Ochi M , Kinoshita K , Yamaguchi JI , Endo H |
| Ref : Xenobiotica , :1 , 2022 |
| Abstract : Ochi_2022_Xenobiotica__1 |
| ESTHER : Ochi_2022_Xenobiotica__1 |
| PubMedSearch : Ochi_2022_Xenobiotica__1 |
| PubMedID: 35296225 |
| Title : Discovery of MGS0274, an ester prodrug of a metabotropic glutamate receptor 2\/3 agonist with improved oral bioavailability - Urabe_2020_Eur.J.Med.Chem_203_112521 |
| Author(s) : Urabe H , Miyakoshi N , Ohtake N , Nozoe A , Ochi M , Fukasawa M , Kinoshita K , Yamaguchi JI , Marumo T , Hikichi H , Chaki S , Hashihayata T |
| Ref : Eur Journal of Medicinal Chemistry , 203 :112521 , 2020 |
| Abstract : Urabe_2020_Eur.J.Med.Chem_203_112521 |
| ESTHER : Urabe_2020_Eur.J.Med.Chem_203_112521 |
| PubMedSearch : Urabe_2020_Eur.J.Med.Chem_203_112521 |
| PubMedID: 32698110 |
| Title : Safety and pharmacokinetic profiles of MGS0274 besylate (TS-134), a novel metabotropic glutamate 2\/3 receptor agonist prodrug, in healthy subjects - Watanabe_2020_Br.J.Clin.Pharmacol_86_2286 |
| Author(s) : Watanabe M , Marcy B , Kinoshita K , Fukasawa M , Hikichi H , Chaki S , Okuyama S , Gevorkyan H , Yoshida S |
| Ref : British Journal of Clinical Pharmacology , 86 :2286 , 2020 |
| Abstract : Watanabe_2020_Br.J.Clin.Pharmacol_86_2286 |
| ESTHER : Watanabe_2020_Br.J.Clin.Pharmacol_86_2286 |
| PubMedSearch : Watanabe_2020_Br.J.Clin.Pharmacol_86_2286 |
| PubMedID: 32353162 |