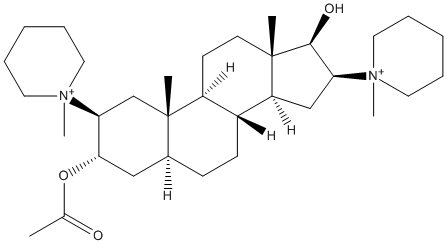
17-desoxypancuronium
Dacuronium, 17-desoxy analog of pancuronium || 17-desoxy analog of pancuronium
General
Type : Nicotinic antagonist,Neuromuscular Nondepolarizing Agents,Not A\/B H target,Acetate,Phenanthrene
Chemical_Nomenclature : [(2S,3S,5S,8R,9S,10S,13S,14S,16S,17R)-17-hydroxy-10,13-dimethyl-2,16-bis(1-methylpiperidin-1-ium-1-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate
Canonical SMILES : CC(=O)OC1CC2CCC3C(C2(CC1[N+]4(CCCCC4)C)C)CCC5(C3CC(C5O)[N+]6(CCCCC6)C)C
InChI : InChI=1S\/C33H58N2O3\/c1-23(36)38-30-20-24-12-13-25-26(33(24,3)22-29(30)35(5)18-10-7-11-19-35)14-15-32(2)27(25)21-28(31(32)37)34(4)16-8-6-9-17-34\/h24-31,37H,6-22H2,1-5H3\/q+2\/t24-,25+,26-,27-,28-,29-,30-,31-,32-,33-\/m0\/s1
InChIKey : XQFBAGUGBNQLRT-ZZZJANDJSA-N || XQFBAGUGBNQLRT-KEEQEOMKSA-N || SUKULMJPMHYWKC-VMDJIQQCSA-L || SUKULMJPMHYWKC-GMMLHHOXSA-L || SUKULMJPMHYWKC-ASCUVKKQSA-L
Other name(s) : 17-desoxypancuronium,Dacuronium,NB68,UNII-VD6L56ZVX1,AC1L54NO,VD6L56ZVX1,CHEMBL3138543,Org 6368
Target
References (3)
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : Differential inhibition of plasma cholinesterase variants using the dibutyrate analogue of pancuronium bromide - Whittaker_1981_Hum.Hered_31_242 |
| Author(s) : Whittaker M , Britten JJ |
| Ref : Hum Hered , 31 :242 , 1981 |
| Abstract : Whittaker_1981_Hum.Hered_31_242 |
| ESTHER : Whittaker_1981_Hum.Hered_31_242 |
| PubMedSearch : Whittaker_1981_Hum.Hered_31_242 |
| PubMedID: 7287015 |