5-pyrid-3-yl-1,3,4-oxadiazole-5e
AChE (IC50 = 50.87 nM) and BuChE (IC50 = 4.77 nM) Reduces lipid peroxidation and glutathione (GSH), decreases beta-amyloid protein
General
Type : Oxadiazol,Pyridine
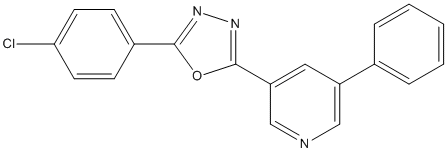
Chemical_Nomenclature : 2-(4-Chlorophenyl)-5-(5-Phenylpyridin)-3-yl-1,3,4-oxadiazole
Canonical SMILES : [C]1=C([C]=N[C]=C1C2=[C][C]=[C][C]=[C]2)C3=NN=C(O3)C4=[C][C]=C([C]=[C]4)Cl
InChI : InChI=1S\/C19H12ClN3O\/c20-17-8-6-14(7-9-17)18-22-23-19(24-18)16-10-15(11-21-12-16)13-4-2-1-3-5-13\/h1-12H
InChIKey : OLPJPJHZTDRYPB-UHFFFAOYSA-N
Other name(s) :
MW : 333.77
Formula : C19H12ClN3O
CAS_number :
PubChem :
UniChem : OLPJPJHZTDRYPB-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 5-pyrid-3-yl-1,3,4-oxadiazole-5e ligand of proteins in family: ACHE || BCHE
Stucture :
Protein : human-ACHE || human-BCHE
References (1)
| Title : Discovery of New 1,3,4-Oxadiazoles with Dual Activity Targeting the Cholinergic Pathway as Effective Anti-Alzheimer Agents - Elghazawy_2022_ACS.Chem.Neurosci__ |
| Author(s) : Elghazawy NH , Zaafar D , Hassan RR , Mahmoud MY , Bedda L , Bakr AF , Arafa RK |
| Ref : ACS Chem Neurosci , : , 2022 |
| Abstract : Elghazawy_2022_ACS.Chem.Neurosci__ |
| ESTHER : Elghazawy_2022_ACS.Chem.Neurosci__ |
| PubMedSearch : Elghazawy_2022_ACS.Chem.Neurosci__ |
| PubMedID: 35377601 |