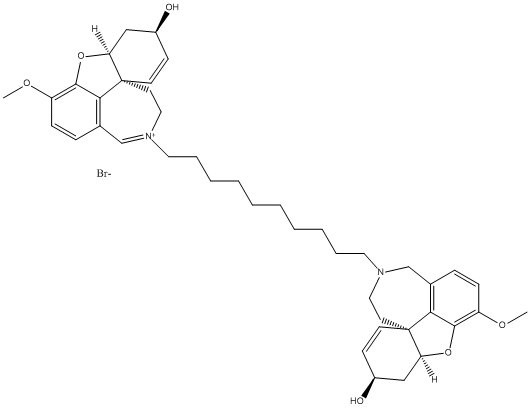
Bis-galanthamine
bis-ligand used to increase AChE inhibition here the compound 6c is represented 10 methylenes linker . The most potent compound described
General
Type : Derivative of Galanthamine,Alkaloid,Alkyl linked bis-ligand,Benzazepin,Azepine,Natural_modified
Chemical_Nomenclature : 6H-benzofuro[3a,3,2-ef][2]benzazepinium, 4a,5,9,10-tetrahydro-6-hydroxy-3-methoxy-11-[10-[(4aS,6R,8aS)-4a,5,9,10-tetrahydro-6-hydroxy-3-methoxy-6H-benzofuro[3a,3,2-ef][2]benzazepin-11(12H)-yl]decyl]-, bromide, (6R,8aS)- (1:1)
Canonical SMILES : COC1=C2C3=C(CN(CCC34C=CC(CC4O2)O)CCCCCCCCCC[N+]5=CC6=C7C(=C(C=C6)OC)OC8C7(CC5)C=CC(C8)O)C=C1.[Br-]
InChI : InChI=1S\/C42H55N2O6.BrH\/c1-47-33-13-11-29-27-43(23-19-41-17-15-31(45)25-35(41)49-39(33)37(29)41)21-9-7-5-3-4-6-8-10-22-44-24-20-42-18-16-32(46)26-36(42)50-40-34(48-2)14-12-30(28-44)38(40)42\;\/h11-18,27,31-32,35-36,45-46H,3-10,19-26,28H2,1-2H3\;1H\/q+1\;\/p-1\/t31-,32-,35?,36-,41-,42-\;\/m0.\/s1
InChIKey : QAMUMNIFAYGMCP-PQXBOIAPSA-M
Other name(s) : CHEMBL415545,(6R,8aS)-6-Hydroxy-11-{10-[(4aS,6R,8aS)-6-hydroxy-3-methoxy-5,6,9,10-tetrahydro-4aH-[1]benzofuro[3a,3,2-ef][2]benzazepin-11(12H)-yl]decyl}-3-methoxy-5,6,9,10-tetrahydro-4aH-[1]benzofuro[3a,3,2-ef][2]b enzazepin-11-ium bromide
MW : 763.81
Formula : C42H56N2O6 Br
CAS_number :
PubChem : 44372540
UniChem : QAMUMNIFAYGMCP-PQXBOIAPSA-M
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Galanthamine as bis-functional ligand for the acetylcholinesterase - Luttmann_2002_J.Mol.Model.(Online)_8_208 |
| Author(s) : Luttmann E , Linnemann E , Fels G |
| Ref : J Mol Model (Online) , 8 :208 , 2002 |
| Abstract : Luttmann_2002_J.Mol.Model.(Online)_8_208 |
| ESTHER : Luttmann_2002_J.Mol.Model.(Online)_8_208 |
| PubMedSearch : Luttmann_2002_J.Mol.Model.(Online)_8_208 |
| PubMedID: 12140604 |
| Title : Potent acetylcholinesterase inhibitors: design, synthesis and structure-activity relationships of alkylene linked bis-galanthamine and galanthamine-galanthaminium salts - Guillou_2000_Bioorg.Med.Chem.Lett_10_637 |
| Author(s) : Guillou C , Mary A , Renko DZ , Gras E , Thal C |
| Ref : Bioorganic & Medicinal Chemistry Lett , 10 :637 , 2000 |
| Abstract : Guillou_2000_Bioorg.Med.Chem.Lett_10_637 |
| ESTHER : Guillou_2000_Bioorg.Med.Chem.Lett_10_637 |
| PubMedSearch : Guillou_2000_Bioorg.Med.Chem.Lett_10_637 |
| PubMedID: 10762042 |