Contilisant
Ligand combining Cholinesterase and Monoamine Oxidase inhibition with Histamine H3 R antagonism for Neurodegenerative Diseases
General
Type : Multitarget,Histamine-Receptor3-H3-Antagonist,Monoamine-oxidase-inhibitor,Piperidine,Indole
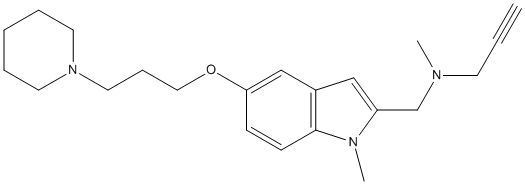
Chemical_Nomenclature : N-Methyl-N-((1-methyl-5-(3-(piperidin-1-yl)propoxy)-1H-indol-2-yl)methyl)prop-2-yn-1-amine
Canonical SMILES : C[N]3C1=C(C=C(C=C1)OCCCN2CCCCC2)C=C3CN(C)CC#C
InChI : InChI=1S\/C22H31N3O\/c1-4-11-23(2)18-20-16-19-17-21(9-10-22(19)24(20)3)26-15-8-14-25-12-6-5-7-13-25\/h1,9-10,16-17H,5-8,11-15,18H2,2-3H3
InChIKey : NQDGCGNKNJWZHP-UHFFFAOYSA-N
Other name(s) :
MW : 353.50
Formula : C22H31N3O
CAS_number :
PubChem : 134817200
UniChem : NQDGCGNKNJWZHP-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Contilisant, a Tetratarget Small Molecule for Alzheimer's Disease Therapy Combining Cholinesterase, Monoamine Oxidase Inhibition, and H3R Antagonism with S1R Agonism Profile - Bautista-Aguilera_2018_J.Med.Chem_61_6937 |
| Author(s) : Bautista-Aguilera OM , Budni J , Mina F , Medeiros EB , Deuther-Conrad W , Entrena JM , Moraleda I , Iriepa I , Lopez-Munoz F , Marco-Contelles J |
| Ref : Journal of Medicinal Chemistry , 61 :6937 , 2018 |
| Abstract : Bautista-Aguilera_2018_J.Med.Chem_61_6937 |
| ESTHER : Bautista-Aguilera_2018_J.Med.Chem_61_6937 |
| PubMedSearch : Bautista-Aguilera_2018_J.Med.Chem_61_6937 |
| PubMedID: 29969030 |
| Title : Multitarget-Directed Ligands Combining Cholinesterase and Monoamine Oxidase Inhibition with Histamine H3 R Antagonism for Neurodegenerative Diseases - Bautista-Aguilera_2017_Angew.Chem.Int.Ed.Engl_56_12765 |
| Author(s) : Bautista-Aguilera OM , Hagenow S , Palomino-Antolin A , Farre-Alins V , Ismaili L , Joffrin PL , Jimeno ML , Soukup O , Janockova J , Kalinowsky L , Proschak E , Iriepa I , Moraleda I , Schwed JS , Romero Martinez A , Lopez-Munoz F , Chioua M , Egea J , Ramsay RR , Marco-Contelles J , Stark H |
| Ref : Angew Chem Int Ed Engl , 56 :12765 , 2017 |
| Abstract : Bautista-Aguilera_2017_Angew.Chem.Int.Ed.Engl_56_12765 |
| ESTHER : Bautista-Aguilera_2017_Angew.Chem.Int.Ed.Engl_56_12765 |
| PubMedSearch : Bautista-Aguilera_2017_Angew.Chem.Int.Ed.Engl_56_12765 |
| PubMedID: 28861918 |