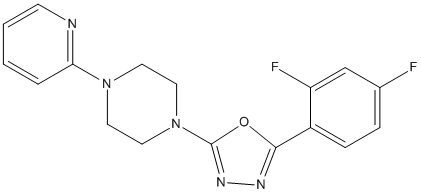
Difluorophenyl-pyridin-piperazin-1,3,4-oxadiazole-49
mixed type of inhibition (Ki = 0.030 microM) (hAChE, IC50 = 0.054 microM), butyrylcholinesterase (hBChE, IC50 = 0.787 microM) and beta-secretase-1 (hBACE-1, IC50 = 0.098 microM).
General
Type : Oxadiazol,Pyridine,Piperazine
Chemical_Nomenclature : 2-(2,4-difluorophenyl)-5-(4-pyridin-2-ylpiperazin-1-yl)-1,3,4-oxadiazole
Canonical SMILES : C1CN(CCN1C2=CC=CC=N2)C3=NN=C(O3)C4=C(C=C(C=C4)F)F
InChI : InChI=1S\/C17H15F2N5O\/c18-12-4-5-13(14(19)11-12)16-21-22-17(25-16)24-9-7-23(8-10-24)15-3-1-2-6-20-15\/h1-6,11H,7-10H2
InChIKey : JDYATWHUSHCNRV-UHFFFAOYSA-N
Other name(s) : Compound 49
MW : 343.12
Formula : C17H15F2N5O
CAS_number :
PubChem : 162657748
UniChem : JDYATWHUSHCNRV-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Difluorophenyl-pyridin-piperazin-1,3,4-oxadiazole-49 ligand of proteins in family: ACHE || BCHE
Stucture :
Protein : human-ACHE || human-BCHE
References (1)
| Title : Design and development of molecular hybrids of 2-pyridylpiperazine and 5-phenyl-1,3,4-oxadiazoles as potential multifunctional agents to treat Alzheimer's disease - Tripathi_2019_Eur.J.Med.Chem_183_111707 |
| Author(s) : Tripathi A , Choubey PK , Sharma P , Seth A , Tripathi PN , Tripathi MK , Prajapati SK , Krishnamurthy S , Shrivastava SK |
| Ref : Eur Journal of Medicinal Chemistry , 183 :111707 , 2019 |
| Abstract : Tripathi_2019_Eur.J.Med.Chem_183_111707 |
| ESTHER : Tripathi_2019_Eur.J.Med.Chem_183_111707 |
| PubMedSearch : Tripathi_2019_Eur.J.Med.Chem_183_111707 |
| PubMedID: 31561043 |