ZLWH-23
anti-AChE potency (IC50 = 0.27 microM) and selective BuChE inhibition (IC50 = 20.82 microM), as well as moderate GSK-3 inhibition (IC50 = 6.78 microM)
General
Type : Piperidine,GSK-3 kinase inhibitor,Carboxamide,Multitarget,Indole,Cyclopropyl,beta-carboline
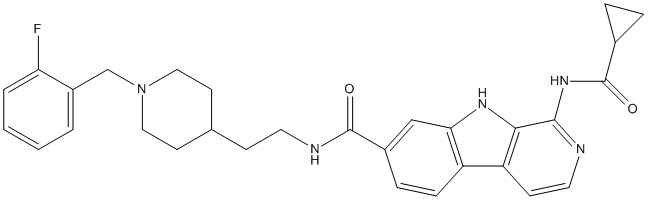
Chemical_Nomenclature : 1-(cyclopropanecarbonylamino)-N-[2-[1-[(2-fluorophenyl)methyl]piperidin-4-yl]ethyl]-9H-pyrido[3,4-b]indole-7-carboxamide
Canonical SMILES : C1CC1C(=O)NC2=NC=CC3=C2NC4=C3C=CC(=C4)C(=O)NCCC5CCN(CC5)CC6=CC=CC=C6F
InChI : InChI=1S\/C30H32FN5O2\/c31-25-4-2-1-3-22(25)18-36-15-11-19(12-16-36)9-13-33-29(37)21-7-8-23-24-10-14-32-28(27(24)34-26(23)17-21)35-30(38)20-5-6-20\/h1-4,7-8,10,14,17,19-20,34H,5-6,9,11-13,15-16,18H2,(H,33,37)(H,32,35,38)
InChIKey : AMLAWNFGPZNOOW-UHFFFAOYSA-N
Other name(s) : HY-144316,CS-0379471
MW : 513.6
Formula : C30H32FN5O2
CAS_number :
PubChem : 163322222
UniChem : AMLAWNFGPZNOOW-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (1)
| Title : Discovery of novel beta-carboline derivatives as selective AChE inhibitors with GSK-3beta inhibitory property for the treatment of Alzheimer's disease - Liu_2021_Eur.J.Med.Chem_229_114095 |
| Author(s) : Liu W , Liu X , Gao Y , Wu L , Huang Y , Chen H , Li D , Zhou L , Wang N , Xu Z , Jiang X , Zhao Q |
| Ref : Eur Journal of Medicinal Chemistry , 229 :114095 , 2021 |
| Abstract : Liu_2021_Eur.J.Med.Chem_229_114095 |
| ESTHER : Liu_2021_Eur.J.Med.Chem_229_114095 |
| PubMedSearch : Liu_2021_Eur.J.Med.Chem_229_114095 |
| PubMedID: 34995924 |