3-Alkylpyridinium-Polymers
Natural inhibitor of AChE from the marine sponge Reniera sarai
General
Type : Polymer,Natural
Chemical_Nomenclature : 3-Alkylpyridinium-Polymers
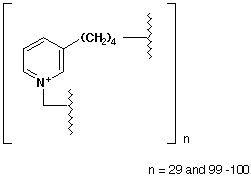
Canonical SMILES :
InChI :
InChIKey :
Other name(s) : polymeric 3-alkylpyridinium salt,nPoly-3-APS,APS12-2
Target
References (23)
| Title : Natural polymeric 3-alkylpyridinium salt affects vertebrate skeletal muscle contractility by preferentially blocking neuromuscular transmission - Zuzek_2017_Toxicol.Lett_281_95 |
| Author(s) : Zuzek MC , Grandic M , Benoit E , Frangez R |
| Ref : Toxicol Lett , 281 :95 , 2017 |
| Abstract : Zuzek_2017_Toxicol.Lett_281_95 |
| ESTHER : Zuzek_2017_Toxicol.Lett_281_95 |
| PubMedSearch : Zuzek_2017_Toxicol.Lett_281_95 |
| PubMedID: 28939337 |
| Title : Pathophysiological Effects of Synthetic Derivatives of Polymeric Alkylpyridinium Salts from the Marine Sponge, Reniera sarai - Grandic_2014_Mar.Drugs_12_2408 |
| Author(s) : Grandic M , Frangez R |
| Ref : Mar Drugs , 12 :2408 , 2014 |
| Abstract : Grandic_2014_Mar.Drugs_12_2408 |
| ESTHER : Grandic_2014_Mar.Drugs_12_2408 |
| PubMedSearch : Grandic_2014_Mar.Drugs_12_2408 |
| PubMedID: 24796301 |
| Title : Antifouling activity of synthetic alkylpyridinium polymers using the barnacle model - Piazza_2014_Mar.Drugs_12_1959 |
| Author(s) : Piazza V , Dragic I , Sepcic K , Faimali M , Garaventa F , Turk T , Berne S |
| Ref : Mar Drugs , 12 :1959 , 2014 |
| Abstract : Piazza_2014_Mar.Drugs_12_1959 |
| ESTHER : Piazza_2014_Mar.Drugs_12_1959 |
| PubMedSearch : Piazza_2014_Mar.Drugs_12_1959 |
| PubMedID: 24699112 |
| Title : APS8, a Polymeric Alkylpyridinium Salt Blocks alpha7 nAChR and Induces Apoptosis in Non-Small Cell Lung Carcinoma - Zovko_2013_Mar.Drugs_11_2574 |
| Author(s) : Zovko A , Viktorsson K , Lewensohn R , Kolosa K , Filipic M , Xing H , Kem WR , Paleari L , Turk T |
| Ref : Mar Drugs , 11 :2574 , 2013 |
| Abstract : Zovko_2013_Mar.Drugs_11_2574 |
| ESTHER : Zovko_2013_Mar.Drugs_11_2574 |
| PubMedSearch : Zovko_2013_Mar.Drugs_11_2574 |
| PubMedID: 23880932 |
| Title : Binding and permeabilization of lipid bilayers by natural and synthetic 3-alkylpyridinium polymers - Grandic_2012_Bioorg.Med.Chem_20_1659 |
| Author(s) : Grandic M , Zovko A , Frangez R , Turk T , Sepcic K |
| Ref : Bioorganic & Medicinal Chemistry , 20 :1659 , 2012 |
| Abstract : Grandic_2012_Bioorg.Med.Chem_20_1659 |
| ESTHER : Grandic_2012_Bioorg.Med.Chem_20_1659 |
| PubMedSearch : Grandic_2012_Bioorg.Med.Chem_20_1659 |
| PubMedID: 22325153 |
| Title : The non-competitive acetylcholinesterase inhibitor APS12-2 is a potent antagonist of skeletal muscle nicotinic acetylcholine receptors - Grandic_2012_Toxicol.Appl.Pharmacol_265_221 |
| Author(s) : Grandic M , Araoz R , Molgo J , Turk T , Sepcic K , Benoit E , Frangez R |
| Ref : Toxicol Appl Pharmacol , 265 :221 , 2012 |
| Abstract : Grandic_2012_Toxicol.Appl.Pharmacol_265_221 |
| ESTHER : Grandic_2012_Toxicol.Appl.Pharmacol_265_221 |
| PubMedSearch : Grandic_2012_Toxicol.Appl.Pharmacol_265_221 |
| PubMedID: 23046821 |
| Title : Chemical synthesis and biological activities of 3-alkyl pyridinium polymeric analogues of marine toxins - Houssen_2010_J.Chem.Biol_3_113 |
| Author(s) : Houssen WE , Lu Z , Edrada-Ebel R , Chatzi C , Tucker SJ , Sepcic K , Turk T , Zovko A , Shen S , Mancini I , Scott RH , Jaspars M |
| Ref : J Chemical Biology , 3 :113 , 2010 |
| Abstract : Houssen_2010_J.Chem.Biol_3_113 |
| ESTHER : Houssen_2010_J.Chem.Biol_3_113 |
| PubMedSearch : Houssen_2010_J.Chem.Biol_3_113 |
| PubMedID: 21326630 |
| Title : Influence of polymeric 3-alkylpyridinium salts from the marine sponge Reniera sarai on the growth of algae and wood decay fungi - Elersek_2008_Biofouling_24_137 |
| Author(s) : Elersek T , Kosi G , Turk T , Pohleven F , Sepcic K |
| Ref : Biofouling , 24 :137 , 2008 |
| Abstract : Elersek_2008_Biofouling_24_137 |
| ESTHER : Elersek_2008_Biofouling_24_137 |
| PubMedSearch : Elersek_2008_Biofouling_24_137 |
| PubMedID: 18274962 |
| Title : Mechanisms of toxicity of 3-alkylpyridinium polymers from marine sponge Reniera sarai - Turk_2007_Mar.Drugs_5_157 |
| Author(s) : Turk T , Frangez R , Sepcic K |
| Ref : Mar Drugs , 5 :157 , 2007 |
| Abstract : Turk_2007_Mar.Drugs_5_157 |
| ESTHER : Turk_2007_Mar.Drugs_5_157 |
| PubMedSearch : Turk_2007_Mar.Drugs_5_157 |
| PubMedID: 18463730 |
| Title : Marine sponge-derived polymeric alkylpyridinium salts as a novel tumor chemotherapeutic targeting the cholinergic system in lung tumors - Paleari_2006_Int.J.Oncol_29_1381 |
| Author(s) : Paleari L , Trombino S , Falugi C , Gallus L , Carlone S , Angelini C , Sepcic K , Turk T , Faimali M , Noonan DM , Albini A |
| Ref : Int J Oncol , 29 :1381 , 2006 |
| Abstract : Paleari_2006_Int.J.Oncol_29_1381 |
| ESTHER : Paleari_2006_Int.J.Oncol_29_1381 |
| PubMedSearch : Paleari_2006_Int.J.Oncol_29_1381 |
| PubMedID: 17088975 |
| Title : Comparative antibacterial activity of polymeric 3-alkylpyridinium salts isolated from the Mediterranean sponge Reniera sarai and their synthetic analogues - Chelossi_2006_Biomol.Eng_23_317 |
| Author(s) : Chelossi E , Mancini I , Sepcic K , Turk T , Faimali M |
| Ref : Biomol Eng , 23 :317 , 2006 |
| Abstract : Chelossi_2006_Biomol.Eng_23_317 |
| ESTHER : Chelossi_2006_Biomol.Eng_23_317 |
| PubMedSearch : Chelossi_2006_Biomol.Eng_23_317 |
| PubMedID: 17113346 |
| Title : Synthesis and bioactivity of linear oligomers related to polymeric alkylpyridinium metabolites from the Mediterranean sponge Reniera sarai - Mancini_2004_Org.Biomol.Chem_2_1368 |
| Author(s) : Mancini I , Sicurelli A , Guella G , Turk T , Macek P , Sepcic K |
| Ref : Org Biomol Chem , 2 :1368 , 2004 |
| Abstract : Mancini_2004_Org.Biomol.Chem_2_1368 |
| ESTHER : Mancini_2004_Org.Biomol.Chem_2_1368 |
| PubMedSearch : Mancini_2004_Org.Biomol.Chem_2_1368 |
| PubMedID: 15105928 |
| Title : N-n-alkylpyridinium analogs, a novel class of nicotinic receptor antagonists: selective inhibition of nicotine-evoked [3H] dopamine overflow from superfused rat striatal slices - Grinevich_2003_J.Pharmacol.Exp.Ther_306_1011 |
| Author(s) : Grinevich VP , Crooks PA , Sumithran SP , Haubner AJ , Ayers JT , Dwoskin LP |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 306 :1011 , 2003 |
| Abstract : Grinevich_2003_J.Pharmacol.Exp.Ther_306_1011 |
| ESTHER : Grinevich_2003_J.Pharmacol.Exp.Ther_306_1011 |
| PubMedSearch : Grinevich_2003_J.Pharmacol.Exp.Ther_306_1011 |
| PubMedID: 12766255 |
| Title : Non-toxic antifouling activity of polymeric 3-alkylpyridinium salts from the Mediterranean sponge Reniera sarai (Pulitzer-Finali) - Faimali_2003_Biofouling_19_47 |
| Author(s) : Faimali M , Sepcic K , Turk T , Geraci S |
| Ref : Biofouling , 19 :47 , 2003 |
| Abstract : Faimali_2003_Biofouling_19_47 |
| ESTHER : Faimali_2003_Biofouling_19_47 |
| PubMedSearch : Faimali_2003_Biofouling_19_47 |
| PubMedID: 14618688 |
| Title : Irreversible and reversible pore formation by polymeric alkylpyridinium salts (poly-APS) from the sponge Reniera sarai - McClelland_2003_Br.J.Pharmacol_139_1399 |
| Author(s) : McClelland D , Evans RM , Abidin I , Sharma S , Choudhry FZ , Jaspars M , Sepcic K , Scott RH |
| Ref : British Journal of Pharmacology , 139 :1399 , 2003 |
| Abstract : McClelland_2003_Br.J.Pharmacol_139_1399 |
| ESTHER : McClelland_2003_Br.J.Pharmacol_139_1399 |
| PubMedSearch : McClelland_2003_Br.J.Pharmacol_139_1399 |
| PubMedID: 12922926 |
| Title : The influence of alkyl pyridinium sponge toxins on membrane properties, cytotoxicity, transfection and protein expression in mammalian cells - Tucker_2003_Biochim.Biophys.Acta_1614_171 |
| Author(s) : Tucker SJ , McClelland D , Jaspars M , Sepcic K , MacEwan DJ , Scott RH |
| Ref : Biochimica & Biophysica Acta , 1614 :171 , 2003 |
| Abstract : Tucker_2003_Biochim.Biophys.Acta_1614_171 |
| ESTHER : Tucker_2003_Biochim.Biophys.Acta_1614_171 |
| PubMedSearch : Tucker_2003_Biochim.Biophys.Acta_1614_171 |
| PubMedID: 12896810 |
| Title : Toxic effects of head-to-tail 3-alkylpyridinium polymers isolated from the marine sponge Reniera sarai in rat - Bunc_2002_Toxicon_40_843 |
| Author(s) : Bunc M , Strupi-Suput J , Vodovnik A , Suput D |
| Ref : Toxicon , 40 :843 , 2002 |
| Abstract : Bunc_2002_Toxicon_40_843 |
| ESTHER : Bunc_2002_Toxicon_40_843 |
| PubMedSearch : Bunc_2002_Toxicon_40_843 |
| PubMedID: 12076636 |
| Title : Interaction of 3-alkylpyridinium polymers from the sea sponge Reniera sarai with insect acetylcholinesterase - Sepcic_1999_J.Protein.Chem_18_251 |
| Author(s) : Sepcic K , Poklar N , Vesnaver G , Fournier D , Turk T , Macek P |
| Ref : J Protein Chem , 18 :251 , 1999 |
| Abstract : Sepcic_1999_J.Protein.Chem_18_251 |
| ESTHER : Sepcic_1999_J.Protein.Chem_18_251 |
| PubMedSearch : Sepcic_1999_J.Protein.Chem_18_251 |
| PubMedID: 10395443 |
| Title : Characterization of hemolytic activity of 3-alkylpyridinium polymers from the marine sponge Reniera sarai - Malovrh_1999_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_124_221 |
| Author(s) : Malovrh P , Sepcic K , Turk T , Macek P |
| Ref : Comparative Biochemistry & Physiology C Pharmacol Toxicol Endocrinol , 124 :221 , 1999 |
| Abstract : Malovrh_1999_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_124_221 |
| ESTHER : Malovrh_1999_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_124_221 |
| PubMedSearch : Malovrh_1999_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_124_221 |
| PubMedID: 10622439 |
| Title : Inhibition of acetylcholinesterase by an alkylpyridinium polymer from the marine sponge, reniera sarai - Sepcic_1998_Biochim.Biophys.Acta_1387_217 |
| Author(s) : Sepcic K , Marcel V , Klaebe A , Turk T , Suput D , Fournier D |
| Ref : Biochimica & Biophysica Acta , 1387 :217 , 1998 |
| Abstract : Sepcic_1998_Biochim.Biophys.Acta_1387_217 |
| ESTHER : Sepcic_1998_Biochim.Biophys.Acta_1387_217 |
| PubMedSearch : Sepcic_1998_Biochim.Biophys.Acta_1387_217 |
| PubMedID: 9748587 |
| Title : Biological activities of aqueous extracts from marine sponges and cytotoxic effects of 3-alkylpyridinium polymers from Reniera sarai - Sepcic_1997_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_117_47 |
| Author(s) : Sepcic K , Batista U , Vacelet J , Macek P , Turk T |
| Ref : Comparative Biochemistry & Physiology C Pharmacology Toxicology & Endocrinology , 117 :47 , 1997 |
| Abstract : Sepcic_1997_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_117_47 |
| ESTHER : Sepcic_1997_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_117_47 |
| PubMedSearch : Sepcic_1997_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_117_47 |
| PubMedID: 9185326 |
| Title : Characterization of anticholinesterase-active 3-alkylpyridinium polymers from the marine sponge Reniera sarai in aqueous solutions - Sepcic_1997_J.Nat.Prod_60_991 |
| Author(s) : Sepcic K , Guella G , Mancini I , Pietra F , Serra MD , Menestrina G , Tubbs K , Macek P , Turk T |
| Ref : Journal of Natural Products , 60 :991 , 1997 |
| Abstract : Sepcic_1997_J.Nat.Prod_60_991 |
| ESTHER : Sepcic_1997_J.Nat.Prod_60_991 |
| PubMedSearch : Sepcic_1997_J.Nat.Prod_60_991 |
| PubMedID: 9358641 |
| Title : Inhibition of acetylcholinesterase by N-alkylpyridinium and N-alkylpyridinium-2-aldoxime salts - |
| Author(s) : De Jong LP , Wolring GZ |
| Ref : Croatica Chemica Acta , 47 :383 , 1975 |
| PubMedID: |