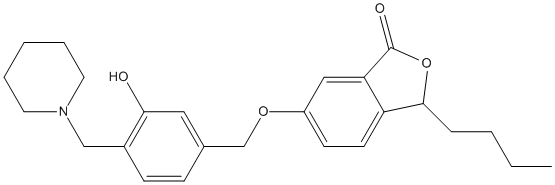
3-butyl-6-benzyloxyphthalide-Mannich-base-7d
3-butyl-6-benzyloxyphthalide Mannich base derivative 7d was the most potent agent with excellent inhibitory activities on EeAChE (IC50 = 0.087 micoM), HuAChE (IC50 = 0.041 microM) and MAO-B (IC50 = 0.30 microM)
General
Type : Monoamine-oxidase-inhibitor,Multitarget,Piperidine,Benzofuran
Chemical_Nomenclature : 3-butyl-6-((3'-hydroxy-4'-(piperidin-1-ylmethyl)benzyl)oxy)isobenzofuran-1(3H)-one
Canonical SMILES : C1=CC(=CC(=C1CN2CCCCC2)O[H])COC3=CC=C4C(=C3)C(OC4CCCC)=O
InChI : InChI=1S\/C26H33NO4\/c1-3-4-8-24-22-12-11-21(16-23(22)26(28)31-24)30-18-19-9-10-20(25(15-19)29-2)17-27-13-6-5-7-14-27\/h9-12,15-16,24H,3-8,13-14,17-18H2,1-2H3
InChIKey : KAFBBPBROARMRS-UHFFFAOYSA-N
Other name(s) :
MW : 409.52
Formula : C25H31NO4
CAS_number :
PubChem :
UniChem : KAFBBPBROARMRS-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 3-butyl-6-benzyloxyphthalide-Mannich-base-7d ligand of proteins in family: ACHE
Stucture :
Protein : human-ACHE
References (1)
| Title : Discovery of novel 3-butyl-6-benzyloxyphthalide Mannich base derivatives as multifunctional agents against Alzheimer's disease - Liu_2022_Bioorg.Med.Chem_58_116660 |
| Author(s) : Liu Z , Shi Y , Zhang X , Yu G , Li J , Cong S , Deng Y |
| Ref : Bioorganic & Medicinal Chemistry , 58 :116660 , 2022 |
| Abstract : Liu_2022_Bioorg.Med.Chem_58_116660 |
| ESTHER : Liu_2022_Bioorg.Med.Chem_58_116660 |
| PubMedSearch : Liu_2022_Bioorg.Med.Chem_58_116660 |
| PubMedID: 35183029 |