4-aminopyridine-1,3,4-oxadiazole-9
non-competitive inhibition (IC50 = 1.098+/-0.140 microM Ki = 0.960 microMhuman-ACHE; IC50 = 7.794+/-0.084 microM human-BCHE)
General
Type : Oxadiazol,Pyridine
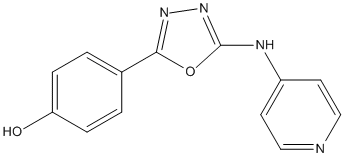
Chemical_Nomenclature : 4-(5-(Pyridin-4-ylamino)-1,3,4-oxadiazol-2-yl)phenol
Canonical SMILES : [C]1=[C]C(=[C][C]=C1[O])C2=NN=C(O2)[N]C3=[C][C]=N[C]=[C]3
InChI : InChI=1S\/C13H10N4O2\/c18-11-3-1-9(2-4-11)12-16-17-13(19-12)15-10-5-7-14-8-6-10\/h1-8,18H,(H,14,15,17)
InChIKey : PDHJMRCHEPBYLZ-UHFFFAOYSA-N
Other name(s) : Compound 9
MW : 254.24
Formula : C13H10N4O2
CAS_number :
PubChem :
UniChem : PDHJMRCHEPBYLZ-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 4-aminopyridine-1,3,4-oxadiazole-9 ligand of proteins in family: ACHE
Stucture :
Protein : human-ACHE
References (1)
| Title : Design and development of 1,3,4-oxadiazole derivatives as potential inhibitors of acetylcholinesterase to ameliorate scopolamine-induced cognitive dysfunctions - Mishra_2019_Bioorg.Chem_89_103025 |
| Author(s) : Mishra P , Sharma P , Tripathi PN , Gupta SK , Srivastava P , Seth A , Tripathi A , Krishnamurthy S , Shrivastava SK |
| Ref : Bioorg Chem , 89 :103025 , 2019 |
| Abstract : Mishra_2019_Bioorg.Chem_89_103025 |
| ESTHER : Mishra_2019_Bioorg.Chem_89_103025 |
| PubMedSearch : Mishra_2019_Bioorg.Chem_89_103025 |
| PubMedID: 31176239 |