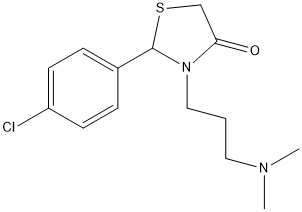
4-thiazolidinone-cpd18
Compound 18 of Vidal-Albalat et al. Specific inhibition of mosquitoe AChE IC50 (microM) AgAChE1 0.86 (0.62-1.2); AaAChE1 0.56 (0.41-0.76); hAChE >200 G122SAgACHE1 >200
General
Type : Thiazolidine,Sulfur Compound
Chemical_Nomenclature : 2-(4-chlorophenyl)-3-[3-(dimethylamino)propyl]-1,3-thiazolidin-4-one
Canonical SMILES : CN(C)CCCN1C(SCC1=O)C2=CC=C(C=C2)Cl
InChI : InChI=1S\/C14H19ClN2OS\/c1-16(2)8-3-9-17-13(18)10-19-14(17)11-4-6-12(15)7-5-11\/h4-7,14H,3,8-10H2,1-2H3
InChIKey : UHOAIESFARWLTF-UHFFFAOYSA-N
Other name(s) : CHEMBL100486,CBMicro_028133,Oprea1_185679,Oprea1_682598,BDBM50002118
MW : 298.8
Formula : C14H19ClN2OS
CAS_number :
PubChem : 2861271
UniChem : UHOAIESFARWLTF-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (1)
| Title : Structure-Activity Relationships Reveal Beneficial Selectivity Profiles of Inhibitors Targeting Acetylcholinesterase of Disease-Transmitting Mosquitoes - Vidal-Albalat_2023_J.Med.Chem__ |
| Author(s) : Vidal-Albalat A , Kindahl T , Rajeshwari R , Lindgren C , Forsgren N , Kitur S , Tengo LS , Ekstrom F , Kamau L , Linusson A |
| Ref : Journal of Medicinal Chemistry , : , 2023 |
| Abstract : Vidal-Albalat_2023_J.Med.Chem__ |
| ESTHER : Vidal-Albalat_2023_J.Med.Chem__ |
| PubMedSearch : Vidal-Albalat_2023_J.Med.Chem__ |
| PubMedID: 37094110 |