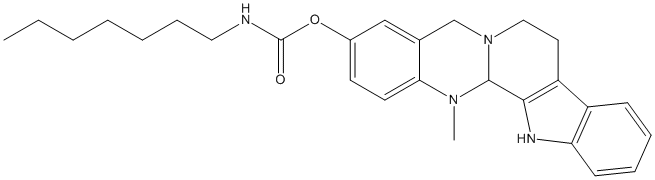
5-deoxo-3-hydroxyevodiamine-heptyl-carbamate
heptyl carbamate of 5-deoxo-3-hydroxyevodiamine || BCHE IC50 77 nM
General
Type : Carbamate,Natural_modified,Derivative of Evodiamine
Chemical_Nomenclature : (21-methyl-3,13,21-triazapentacyclo[11.8.0.02,10.04,9.015,20]henicosa-2(10),4,6,8,15(20),16,18-heptaen-17-yl) N-heptylcarbamate
Canonical SMILES : CCCCCCCNC(=O)OC1=CC2=C(C=C1)N(C3C4=C(CCN3C2)C5=CC=CC=C5N4)C
InChI : InChI=1S\/C27H34N4O2\/c1-3-4-5-6-9-15-28-27(32)33-20-12-13-24-19(17-20)18-31-16-14-22-21-10-7-8-11-23(21)29-25(22)26(31)30(24)2\/h7-8,10-13,17,26,29H,3-6,9,14-16,18H2,1-2H3,(H,28,32)
InChIKey : HKHKOTPYNYYBTG-UHFFFAOYSA-N
Other name(s) : CHEMBL3262489,BDBM50016244,Compound 11c
MW : 446.6
Formula : C27H34N4O2
CAS_number :
PubChem : 90643589
UniChem : HKHKOTPYNYYBTG-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 5-deoxo-3-hydroxyevodiamine-heptyl-carbamate ligand of proteins in family: BCHE
Stucture :
Protein : human-BCHE
References (1)
| Title : Identification of a neuroprotective and selective butyrylcholinesterase inhibitor derived from the natural alkaloid evodiamine - Huang_2014_Eur.J.Med.Chem_81C_15 |
| Author(s) : Huang G , Kling B , Darras FH , Heilmann J , Decker M |
| Ref : Eur Journal of Medicinal Chemistry , 81C :15 , 2014 |
| Abstract : Huang_2014_Eur.J.Med.Chem_81C_15 |
| ESTHER : Huang_2014_Eur.J.Med.Chem_81C_15 |
| PubMedSearch : Huang_2014_Eur.J.Med.Chem_81C_15 |
| PubMedID: 24819955 |