6AX1-cp3
Oxadiazole 3-substituted azetidine carbamate IC50 0.38nM for MGLL
General
Type : Halo ketone,Trifluoro,Carbamate,Azetidin,Oxadiazol
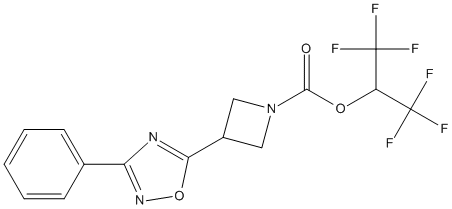
Chemical_Nomenclature : 1,1,1,3,3,3-Hexafluoropropan-2-yl 3-(3-Phenyl-1,2,4-oxadiazol-5-yl)azetidine-1-carboxylate
Canonical SMILES : FC(F)(F)C(OC(=O)N1CC(C1)c1nc(no1)-c1ccccc1)C(F)(F)F
InChI : InChI=1S\/C15H11F6N3O3\/c16-14(17,18)12(15(19,20)21)26-13(25)24-6-9(7-24)11-22-10(23-27-11)8-4-2-1-3-5-8\/h1-5,9,12H,6-7H2
InChIKey : MQSOFDLFKHBDFY-UHFFFAOYSA-N
Other name(s) : CHEMBL4089505,C0S
MW : 395.26
Formula : C15H11F6N3O3
CAS_number :
PubChem : 137349039
UniChem : MQSOFDLFKHBDFY-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 6AX1-cp3 ligand of proteins in family: Monoglyceridelipase_lysophospholip
Stucture : 6AX1 Structure of human monoacylglycerol lipase bound to a covalent inhibitor
Protein : human-MGLL
References (1)
| Title : Azetidine and Piperidine Carbamates as Efficient, Covalent Inhibitors of Monoacylglycerol Lipase - Butler_2017_J.Med.Chem_60_9860 |
| Author(s) : Butler CR , Beck EM , Harris A , Huang Z , McAllister LA , Am Ende CW , Fennell K , Foley TL , Fonseca K , Hawrylik SJ , Johnson DS , Knafels JD , Mente S , Noell GS , Pandit J , Phillips TB , Piro JR , Rogers BN , Samad TA , Wang J , Wan S , Brodney MA |
| Ref : Journal of Medicinal Chemistry , 60 :9860 , 2017 |
| Abstract : Butler_2017_J.Med.Chem_60_9860 |
| ESTHER : Butler_2017_J.Med.Chem_60_9860 |
| PubMedSearch : Butler_2017_J.Med.Chem_60_9860 |
| PubMedID: 29148769 |
| Gene_locus related to this paper: human-MGLL |