6E6T-HVV
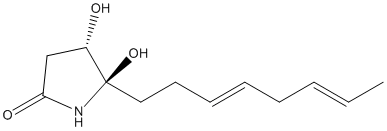
Product of epoxide ring opening of cerulenin-hemiaminal by NcmC. Cerulenin is an epoxydodecadienamide isolated from several fungi species, including Acremonium, Acrocylindrum and Helicoceras Cephalosporium caerulens. It inhibits the biosynthesis of several lipids by interfering with enzyme function. It has a role as an antifungal agent, an antiinfective agent, an antilipemic drug, an antimetabolite, a fatty acid synthesis inhibitor and an antimicrobial agent. It is a monocarboxylic acid amide and an epoxide.In fatty acid synthesis inhibits b-keto-acyl-ACP synthase. In sterol synthesis, inhibits HMG-CoA synthetase activity. Interconversion of cerulenin from its linear to cyclic hemiaminal form,occurs in polar protic solvents. It is this hemiaminal form which is substrate of epoxide ring opening by NcmC
General
Type : Pyrrolidine
Chemical_Nomenclature : (4S,5R)-4,5-Dihydroxy-5-[(3E,6E)-octa-3,6-dienyl]pyrrolidin-2-one
Canonical SMILES : CC=CCC=CCCC1(C(CC(=O)N1)O)O
InChI : InChI=1S\/C12H19NO3\/c1-2-3-4-5-6-7-8-12(16)10(14)9-11(15)13-12\/h2-3,5-6,10,14,16H,4,7-9H2,1H3,(H,13,15)\/b3-2+,6-5+\/t10-,12+\/m0\/s1
InChIKey : VIMHNMLESUAQPL-JFDNTZKISA-N
Other name(s) : HVV
MW : 225.28
Formula : C12H19NO3
CAS_number :
PubChem : 138857404
UniChem : VIMHNMLESUAQPL-JFDNTZKISA-N
IUPHAR :
Wikipedia :
Target
Families : 6E6T-HVV ligand of proteins in family: Dieckmann_Cyclase
Stucture : 6E6T Dieckmann cyclase from Saccharothrix syringae, NcmC, bound to cerulenin
Protein : 9pseu-NcmC
References (1)
| Title : Structural Basis for Enzymatic Off-Loading of Hybrid Polyketides by Dieckmann Condensation - Cogan_2020_ACS.Chem.Biol_15_2783 |
| Author(s) : Cogan DP , Ly J , Nair SK |
| Ref : ACS Chemical Biology , 15 :2783 , 2020 |
| Abstract : Cogan_2020_ACS.Chem.Biol_15_2783 |
| ESTHER : Cogan_2020_ACS.Chem.Biol_15_2783 |
| PubMedSearch : Cogan_2020_ACS.Chem.Biol_15_2783 |
| PubMedID: 33017142 |
| Gene_locus related to this paper: 9pseu-NcmC |