AEPU
General
Type : Adamantyl,Urea derivative
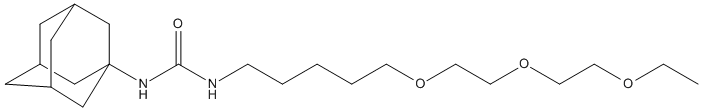
Chemical_Nomenclature : 1-(1-adamantyl)-3-[5-[2-(2-ethoxyethoxy)ethoxy]pentyl]urea
Canonical SMILES : CCOCCOCCOCCCCCNC(=O)NC12CC3CC(C1)CC(C3)C2
InChI : InChI=1S\/C22H40N2O4\/c1-2-26-8-9-28-11-10-27-7-5-3-4-6-23-21(25)24-22-15-18-12-19(16-22)14-20(13-18)17-22\/h18-20H,2-17H2,1H3,(H2,23,24,25)
InChIKey : NBKGUPAYHLZPHD-UHFFFAOYSA-N
Other name(s) : 1-adamantanyl-3-{5-[2-(2-ethylethoxy)ethoxy]pentyl]}urea,CHEMBL242655,Urea-based compound, 19,SCHEMBL231301,SCHEMBL14621913,BDBM25738
MW : 396.6
Formula : C22H40N2O4
CAS_number :
PubChem : 11280933
UniChem : NBKGUPAYHLZPHD-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (3)
| Title : In vitro and in vivo metabolism of N-adamantyl substituted urea-based soluble epoxide hydrolase inhibitors - Liu_2015_Biochem.Pharmacol_98_718 |
| Author(s) : Liu JY , Tsai HJ , Morisseau C , Lango J , Hwang SH , Watanabe T , Kim IH , Hammock BD |
| Ref : Biochemical Pharmacology , 98 :718 , 2015 |
| Abstract : Liu_2015_Biochem.Pharmacol_98_718 |
| ESTHER : Liu_2015_Biochem.Pharmacol_98_718 |
| PubMedSearch : Liu_2015_Biochem.Pharmacol_98_718 |
| PubMedID: 26494425 |
| Title : Screening a library of 1600 adamantyl ureas for anti-Mycobacterium tuberculosis activity in vitro and for better physical chemical properties for bioavailability - Scherman_2012_Bioorg.Med.Chem_20_3255 |
| Author(s) : Scherman MS , North EJ , Jones V , Hess TN , Grzegorzewicz AE , Kasagami T , Kim IH , Merzlikin O , Lenaerts AJ , Lee RE , Jackson M , Morisseau C , McNeil MR |
| Ref : Bioorganic & Medicinal Chemistry , 20 :3255 , 2012 |
| Abstract : Scherman_2012_Bioorg.Med.Chem_20_3255 |
| ESTHER : Scherman_2012_Bioorg.Med.Chem_20_3255 |
| PubMedSearch : Scherman_2012_Bioorg.Med.Chem_20_3255 |
| PubMedID: 22522007 |
| Title : Inhibition of soluble epoxide hydrolase does not protect against endotoxin-mediated hepatic inflammation - Fife_2008_J.Pharmacol.Exp.Ther_327_707 |
| Author(s) : Fife KL , Liu Y , Schmelzer KR , Tsai HJ , Kim IH , Morisseau C , Hammock BD , Kroetz DL |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 327 :707 , 2008 |
| Abstract : Fife_2008_J.Pharmacol.Exp.Ther_327_707 |
| ESTHER : Fife_2008_J.Pharmacol.Exp.Ther_327_707 |
| PubMedSearch : Fife_2008_J.Pharmacol.Exp.Ther_327_707 |
| PubMedID: 18815352 |