AS115
racemic AS 115 is a potent and selective inactivator of human-NCEH1 (KIAA1363, AADACL1), displaying an IC50 value of 150 nM
General
Type : Carbamate
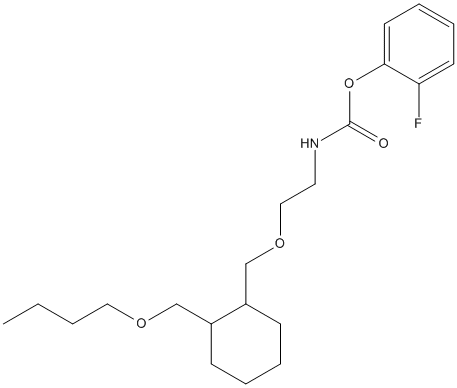
Chemical_Nomenclature : (2-fluorophenyl) N-[2-[[2-(butoxymethyl)cyclohexyl]methoxy]ethyl]carbamate
Canonical SMILES : CCCCOCC1CCCCC1COCCNC(=O)OC2=CC=CC=C2F
InChI : InChI=1S\/C21H32FNO4\/c1-2-3-13-25-15-17-8-4-5-9-18(17)16-26-14-12-23-21(24)27-20-11-7-6-10-19(20)22\/h6-7,10-11,17-18H,2-5,8-9,12-16H2,1H3,(H,23,24)
InChIKey : BTYYXSHZANWPEB-UHFFFAOYSA-N
Other name(s) : CHEMBL2069333,C21H32FNO4,1002AH,BDBM50390508,ZINC44606711,SCHEMBL13912213,LP047347,(+)-AS 115,(-)-AS 115
MW : 381.48
Formula : C21H32FNO4
CAS_number : 926657-43-4
UniChem : BTYYXSHZANWPEB-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (1)
| Title : An enzyme that regulates ether lipid signaling pathways in cancer annotated by multidimensional profiling - Chiang_2006_Chem.Biol_13_1041 |
| Author(s) : Chiang KP , Niessen S , Saghatelian A , Cravatt BF |
| Ref : Chemical Biology , 13 :1041 , 2006 |
| Abstract : Chiang_2006_Chem.Biol_13_1041 |
| ESTHER : Chiang_2006_Chem.Biol_13_1041 |
| PubMedSearch : Chiang_2006_Chem.Biol_13_1041 |
| PubMedID: 17052608 |
| Gene_locus related to this paper: human-NCEH1 , mouse-Q8BLF1 |