Aflatoxin-B1
A potent hepatotoxic and hepatocarcinogenic mycotoxin produced by the Aspergillus flavus group of fungi. It is also mutagenic, teratogenic, and causes immunosuppression in animals. It is found as a contaminant in peanuts, cottonseed meal, corn, and other grains. The mycotoxin requires epoxidation to aflatoxin B1 2,3-oxide for activation. Microsomal monooxygenases biotransform the toxin to the less toxic metabolites aflatoxin M1 and Q1
General
Type : Natural,Toxin,Mycotoxin-metabolite
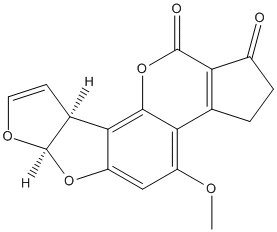
Chemical_Nomenclature : 2,3,6a,9a-tetrahydro-4-methoxycyclopenta(c)furo(3',2':4,5)furo(2,3-h)(1)benzo-pyran-1,11-dione
Canonical SMILES : COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C5C=COC5OC4=C1
InChI : InChI=1S\/C17H12O6\/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15\/h4-6,8,17H,2-3H2,1H3\/t8-,17+\/m0\/s1
InChIKey : OQIQSTLJSLGHID-WNWIJWBNSA-N
Other name(s) : NSC 529592,NSC-529592,CCRIS 12
MW : 312.27
Formula : C17H12O6
CAS_number : 1162-65-8
PubChem : 186907
UniChem : OQIQSTLJSLGHID-WNWIJWBNSA-N
IUPHAR :
Wikipedia : Aflatoxin
Target
Families : Aflatoxin-B1 ligand of proteins in family: ACHE
Stucture : 2XI4 Torpedo californica Acetylcholinesterase in Complex with Aflatoxin B1 (Orthorhombic Form)
Protein : torca-ACHE
References (2)
| Title : Molecular modeling studies on the interactions of aflatoxin B1 and its metabolites with the peripheral anionic site (PAS) of human acetylcholinesterase - de Almeida_2018_J.Biomol.Struct.Dyn__1 |
| Author(s) : de Almeida JSFD , Cavalcante SFA , Dolezal R , Kuca K , Musilek K , Jun D , Franca TCC |
| Ref : J Biomol Struct Dyn , :1 , 2018 |
| Abstract : de Almeida_2018_J.Biomol.Struct.Dyn__1 |
| ESTHER : de Almeida_2018_J.Biomol.Struct.Dyn__1 |
| PubMedSearch : de Almeida_2018_J.Biomol.Struct.Dyn__1 |
| PubMedID: 29749305 |
| Title : Backdoor opening mechanism in acetylcholinesterase based on X-ray crystallography and molecular dynamics simulations - Sanson_2011_Protein.Sci_20_1114 |
| Author(s) : Sanson B , Colletier JP , Xu Y , Lang PT , Jiang H , Silman I , Sussman JL , Weik M |
| Ref : Protein Science , 20 :1114 , 2011 |
| Abstract : Sanson_2011_Protein.Sci_20_1114 |
| ESTHER : Sanson_2011_Protein.Sci_20_1114 |
| PubMedSearch : Sanson_2011_Protein.Sci_20_1114 |
| PubMedID: 21594947 |
| Gene_locus related to this paper: torca-ACHE |