Amiloride
A pyrazine compound inhibiting sodium reabsorption through sodium channels in renal epithelial cells. This inhibition creates a negative potential in the luminal membranes of principal cells, located in the distal convoluted tubule and collecting duct. Negative potential reduces secretion of potassium and hydrogen ions. Amiloride is used in conjunction with diuretics to spare potassium loss. (From Gilman et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 9th ed, p705). Inhibition or activation of cholinesterases depending on substrates
General
Type : Carboxamide,Drug,Pyridine,Not A\/B H target
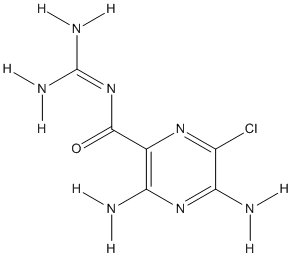
Chemical_Nomenclature : 3,5-diamino-6-chloro-N-(diaminomethylidene)pyrazine-2-carboxamide
Canonical SMILES : C1(=C(N=C(C(=N1)Cl)N)N)C(=O)N=C(N)N
InChI : InChI=1S\/C6H8ClN7O\/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11\/h(H4,8,9,13)(H4,10,11,14,15)
InChIKey : XSDQTOBWRPYKKA-UHFFFAOYSA-N
Other name(s) : 3,5-diamino-N-(aminoiminomethyl)-6-chloropyrazineearboxamide,Midamor,Amipramidin,Guanamprazine,Amipramizid,Amipramizide
MW : 229.62
Formula : C6H8ClN7O
CAS_number :
PubChem : 16231
UniChem : XSDQTOBWRPYKKA-UHFFFAOYSA-N
IUPHAR : 2421
Wikipedia : Amiloride
Target
References (3)
| Title : Substrate dependence of amiloride- and soman-induced conformation changes of butyrylcholinesterase as evidenced by high-pressure perturbation - Clery_1995_Biochim.Biophys.Acta_1250_19 |
| Author(s) : Clery C , Heiber-Langer I , Channac L , David L , Balny C , Masson P |
| Ref : Biochimica & Biophysica Acta , 1250 :19 , 1995 |
| Abstract : Clery_1995_Biochim.Biophys.Acta_1250_19 |
| ESTHER : Clery_1995_Biochim.Biophys.Acta_1250_19 |
| PubMedSearch : Clery_1995_Biochim.Biophys.Acta_1250_19 |
| PubMedID: 7612649 |
| Title : The interaction of amiloride with acetylcholinesterase and butyrylcholinesterase - Zemach_1990_FEBS.Lett_263_166 |
| Author(s) : Zemach L , Segal D , Shalitin Y |
| Ref : FEBS Letters , 263 :166 , 1990 |
| Abstract : Zemach_1990_FEBS.Lett_263_166 |
| ESTHER : Zemach_1990_FEBS.Lett_263_166 |
| PubMedSearch : Zemach_1990_FEBS.Lett_263_166 |
| PubMedID: 2332047 |
| Title : Amiloride is a cholinergic antagonist in the rabbit pancreas - Kuijpers_1984_Biochim.Biophys.Acta_804_237 |
| Author(s) : Kuijpers GA , De Pont JJ , Van Nooy IG , Fleuren-Jakobs AM , Bonting SL , Rodrigues de Miranda JF |
| Ref : Biochimica & Biophysica Acta , 804 :237 , 1984 |
| Abstract : Kuijpers_1984_Biochim.Biophys.Acta_804_237 |
| ESTHER : Kuijpers_1984_Biochim.Biophys.Acta_804_237 |
| PubMedSearch : Kuijpers_1984_Biochim.Biophys.Acta_804_237 |
| PubMedID: 6202328 |