Bis-Thiazolidinone-Based-20
ACHE IC50 = 0.070 +/-0.050 microM, BCHE IC50 = 0.10+/-0.050 microM
General
Type : Thiazolidine,Sulfur Compound
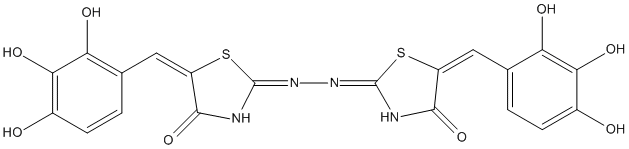
Chemical_Nomenclature : 2-((4-oxo-5-((E)-2,3,4-trihydroxybenzylidene)thiazolidin-2-ylidene)hydrazono)-5-((E)-3,4,5-trihydroxybenzylidene)thiazolidin-4-one
Canonical SMILES : C3(=NN=C1NC(C(S1)=CC2=CC=C(C(=C2O)O)O)=O)SC(C(N3)=O)=CC4=C(C(=C(C=C4)O)O)O
InChI : InChI=1S\/C20H14N4O8S2\/c25-9-3-1-7(13(27)15(9)29)5-11-17(31)21-19(33-11)23-24-20-22-18(32)12(34-20)6-8-2-4-10(26)16(30)14(8)28\/h1-6,25-30H,(H,21,23,31)(H,22,24,32)\/b11-5-,12-6+
InChIKey : MSQZMILSECVAJQ-JTAXDKCCSA-N
Other name(s) :
MW : 502.47
Formula : C20H14N4O8S2
CAS_number :
PubChem :
UniChem : MSQZMILSECVAJQ-JTAXDKCCSA-N
IUPHAR :
Wikipedia :
Target
Families : Bis-Thiazolidinone-Based-20 ligand of proteins in family: ACHE || BCHE
Stucture :
Protein :
References (1)
| Title : New bis-thiazolidinone based chalcone analogues as effective inhibitors of Alzheimer's disease: Synthesis, molecular docking, acetylcholinesterase and butyrylcholinesterase study - Hussain_2022_Chem.Biodivers__ |
| Author(s) : Hussain R , Rahim F , Rehman W , Taha M , Khan S , Zaman K , Ali Shah SA , Wadood A , Imran S , Abdellatif M |
| Ref : Chem Biodivers , : , 2022 |
| Abstract : Hussain_2022_Chem.Biodivers__ |
| ESTHER : Hussain_2022_Chem.Biodivers__ |
| PubMedSearch : Hussain_2022_Chem.Biodivers__ |
| PubMedID: 35997224 |