BisQ
Photoswitchable agonist of the nicotinic acetylcholine receptor developped by Ehrlanger and Lester( trans most active form). BisQ is a poor inhibitor of AChE Ki 3129.3+/-1181.4 nM. Has no photodependent effect on AChE activity
General
Type : Analogue of substrate,Trimethylammonium,Bisquaternary,Not A\/B H target,Nicotinic agonist,Photoswitch
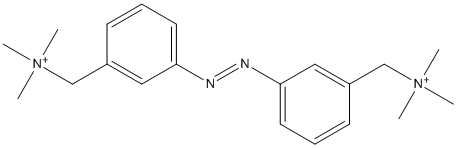
Chemical_Nomenclature : trimethyl-[[4-[[4-[(trimethylazaniumyl)methyl]phenyl]diazenyl]phenyl]methyl]azanium
Canonical SMILES : C[N+](C)(C)CC1=CC=C(C=C1)N=NC2=CC=C(C=C2)C[N+](C)(C)C
InChI : InChI=1S\/C20H30N4\/c1-23(2,3)15-17-7-11-19(12-8-17)21-22-20-13-9-18(10-14-20)16-24(4,5)6\/h7-14H,15-16H2,1-6H3\/q+2
InChIKey : BUBUVNULLZBOQS-UHFFFAOYSA-N
Other name(s) : 4,4'-Bis(trimethylaminiomethyl)azobenzene
MW : 326.5
Formula : C20H30N4+2
CAS_number :
PubChem : 14895427, 133795, 123131939
UniChem : BUBUVNULLZBOQS-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (4)
| Title : Optical control of acetylcholinesterase with a tacrine switch - Broichhagen_2014_Angew.Chem.Int.Ed.Engl_53_7657 |
| Author(s) : Broichhagen J , Jurastow I , Iwan K , Kummer W , Trauner D |
| Ref : Angew Chem Int Ed Engl , 53 :7657 , 2014 |
| Abstract : Broichhagen_2014_Angew.Chem.Int.Ed.Engl_53_7657 |
| ESTHER : Broichhagen_2014_Angew.Chem.Int.Ed.Engl_53_7657 |
| PubMedSearch : Broichhagen_2014_Angew.Chem.Int.Ed.Engl_53_7657 |
| PubMedID: 24895330 |
| Title : Physiological and pharmacological manipulations with light flashes - |
| Author(s) : Lester HA , Nerbonne JM |
| Ref : Annu Rev Biophys Bioeng , 11 :151 , 1982 |
| PubMedID: 7049061 |
| Title : Electrophysiological experiments with photoisomerizable cholinergic compounds: review and progress report - |
| Author(s) : Lester HA , Nass MM , Krouse ME , Nerbonne JM , Wassermann NH , Erlanger BF |
| Ref : Annals of the New York Academy of Sciences , 346 :475 , 1980 |
| PubMedID: 6247953 |
| Title : Photochromic activators of the acetylcholine receptor - Bartels_1971_Proc.Natl.Acad.Sci.U.S.A_68_1820 |
| Author(s) : Bartels E , Wassermann NH , Erlanger BF |
| Ref : Proc Natl Acad Sci U S A , 68 :1820 , 1971 |
| Abstract : Bartels_1971_Proc.Natl.Acad.Sci.U.S.A_68_1820 |
| ESTHER : Bartels_1971_Proc.Natl.Acad.Sci.U.S.A_68_1820 |
| PubMedSearch : Bartels_1971_Proc.Natl.Acad.Sci.U.S.A_68_1820 |
| PubMedID: 5288770 |