CHEMBL1951424
IC50: 17 nM
General
Type : Piperidine,Cyanide,Quinoline,Pyrrolidine
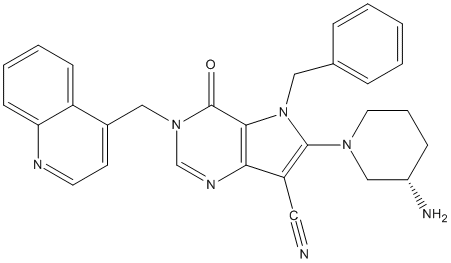
Chemical_Nomenclature : 6-[(3S)-3-aminopiperidin-1-yl]-5-benzyl-4-oxo-3-(quinolin-4-ylmethyl)pyrrolo[3,2-d]pyrimidine-7-carbonitrile
Canonical SMILES : C1CC(CN(C1)C2=C(C3=C(N2CC4=CC=CC=C4)C(=O)N(C=N3)CC5=CC=NC6=CC=CC=C56)C#N)N
InChI : InChI=1S\/C29H27N7O\/c30-15-24-26-27(29(37)35(19-33-26)17-21-12-13-32-25-11-5-4-10-23(21)25)36(16-20-7-2-1-3-8-20)28(24)34-14-6-9-22(31)18-34\/h1-5,7-8,10-13,19,22H,6,9,14,16-18,31H2\/t22-\/m0\/s1
InChIKey : WRJSBPQWADEDBH-QFIPXVFZSA-N
Other name(s) : SCHEMBL4225060,BDBM50364164,6-[(3s)-3-Aminopiperidin-1-Yl]-5-Benzyl-4-Oxo-3-(Quinolin-4-Ylmethyl)-4,5-Dihydro-3h-Pyrrolo[3,2-D]pyrimidine-7-Carbonitrile,N7F
MW : 489.58
Formula : C29H27N7O
CAS_number :
PubChem : 23633348
UniChem : WRJSBPQWADEDBH-QFIPXVFZSA-N
IUPHAR :
Wikipedia :
Target
Families : CHEMBL1951424 ligand of proteins in family: DPP4N_Peptidase_S9
Stucture : 4A5S Crystal Structure of human DDP4 in complex with a novel heterocyclic DPP4 inhibitor
Protein : human-DPP4
References (1)
| Title : Novel heterocyclic DPP-4 inhibitors for the treatment of type 2 diabetes. - Sutton_2012_Bioorg.Med.Chem.Lett_22_1464 |
| Author(s) : Sutton JM , Clark DE , Dunsdon SJ , Fenton G , Fillmore A , Harris NV , Higgs C , Hurley CA , Krintel SL , Mackenzie RE , Duttaroy A , Gangl E , Maniara W , Sedrani R , Namoto K , Ostermann N , Gerhartz B , Sirockin F , Trappe J , Hassiepen U , Baeschlin DK |
| Ref : Bioorganic & Medicinal Chemistry Lett , 22 :1464 , 2012 |
| Abstract : Sutton_2012_Bioorg.Med.Chem.Lett_22_1464 |
| ESTHER : Sutton_2012_Bioorg.Med.Chem.Lett_22_1464 |
| PubMedSearch : Sutton_2012_Bioorg.Med.Chem.Lett_22_1464 |
| PubMedID: 22177783 |
| Gene_locus related to this paper: human-DPP4 |