CHEMBL4079247
Leaves the adduct Methyl dimethylcarbamate on the active serine
General
Type : Carbamate
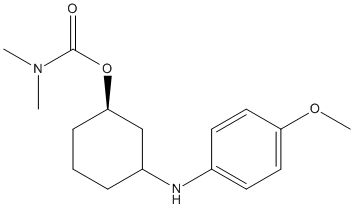
Chemical_Nomenclature : [(1R,3R)-3-(4-methoxyanilino)cyclohexyl] N,N-dimethylcarbamate
Canonical SMILES : CN(C)C(=O)OC1CCCC(C1)NC2=CC=C(C=C2)OC
InChI : InChI=1S\/C16H24N2O3\/c1-18(2)16(19)21-15-6-4-5-13(11-15)17-12-7-9-14(20-3)10-8-12\/h7-10,13,15,17H,4-6,11H2,1-3H3\/t13-,15-\/m1\/s1
InChIKey : DJUUOKKQPMZYKO-UKRRQHHQSA-N
Other name(s) : BDBM50245735,CHEMBL4080162,BDBM50245729,trans-3-(4-Methoxyphenylamino)cyclohexyl N,N-dimethylcarbamate hydrochloride,6c',5c'
MW : 292.37
Formula : C16H24N2O3\
CAS_number :
PubChem : 137651291
UniChem : DJUUOKKQPMZYKO-UKRRQHHQSA-N
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer's disease - Li_2017_Eur.J.Med.Chem_132_294 |
| Author(s) : Li Q , Yang H , Chen Y , Sun H |
| Ref : Eur Journal of Medicinal Chemistry , 132 :294 , 2017 |
| Abstract : Li_2017_Eur.J.Med.Chem_132_294 |
| ESTHER : Li_2017_Eur.J.Med.Chem_132_294 |
| PubMedSearch : Li_2017_Eur.J.Med.Chem_132_294 |
| PubMedID: 28371641 |
| Title : Cholinesterases Inhibition by Novel cis- and trans-3-Arylaminocyclohexyl N, N-Dimethylcarbamates: Biological Evaluation and Molecular Modeling - Yamazaki_2016_J.Braz.Chem.Soc_27_1616 |
| Author(s) : Yamazaki DA , Candido AA , Bagatin MC , Machinski M, Jr. , Mossini SAG , Pontes RM , Rosa FA , Bassoa EA , Gauze GF |
| Ref : J Braz Chem Soc , 27 :1616 , 2016 |
| Abstract : Yamazaki_2016_J.Braz.Chem.Soc_27_1616 |
| ESTHER : Yamazaki_2016_J.Braz.Chem.Soc_27_1616 |
| PubMedSearch : Yamazaki_2016_J.Braz.Chem.Soc_27_1616 |
| PubMedID: |