Chinese-VX
Similar to VX
General
Type : Chemical Warfare Agent,Organophosphate,Nerve Agent V-series,Sulfur Compound
Chemical_Nomenclature : 2-[butoxy(methyl)phosphoryl]sulfanyl-N,N-diethylethanamine
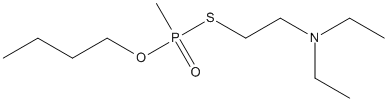
Canonical SMILES : CCCCOP(=O)(C)SCCN(CC)CC
InChI : InChI=1S\/C11H26NO2PS\/c1-5-8-10-14-15(4,13)16-11-9-12(6-2)7-3\/h5-11H2,1-4H3
InChIKey : BTVFLPKWKJBKNN-UHFFFAOYSA-N
Other name(s) : AC1LBKUK,O-(n-Butyl) S-[2-(diethylamino)ethyl]-methanephosphonothioate,2-[butoxy(methyl)phosphoryl]sulfanyl-N,N-diethylethanamine,O-(n-Butyl) S-(2-diethylaminoethyl) methylphosphonothiolate,Phosphonothioic acid, methyl-, O-butyl S-[2-(diethylamino)ethyl] ester,CVX,EA-6043
MW : 267,37
Formula : C11H26NO2PS
CAS_number : 468712-10-9
PubChem : 559704
UniChem : BTVFLPKWKJBKNN-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Chinese-VX ligand of proteins in family: ACHE || BCHE
Stucture : 2XQI X-ray Structure of human butyrylcholinesterase inhibited by racemic CVX
Protein : human-BCHE
References (5)
| Title : The prediction of acute toxicity (LD(50)) for organophosphorus-based chemical warfare agents (V-series) using toxicology in silico methods - Noga_2023_Arch.Toxicol__ |
| Author(s) : Noga M , Michalska A , Jurowski K |
| Ref : Archives of Toxicology , : , 2023 |
| Abstract : Noga_2023_Arch.Toxicol__ |
| ESTHER : Noga_2023_Arch.Toxicol__ |
| PubMedSearch : Noga_2023_Arch.Toxicol__ |
| PubMedID: 38051368 |
| Title : V-type nerve agents phosphonylate ubiquitin at biologically relevant lysine residues and induce intramolecular cyclization by an isopeptide bond - Schmidt_2014_Anal.Bioanal.Chem_406_5171 |
| Author(s) : Schmidt C , Breyer F , Blum MM , Thiermann H , Worek F , John H |
| Ref : Anal Bioanal Chem , 406 :5171 , 2014 |
| Abstract : Schmidt_2014_Anal.Bioanal.Chem_406_5171 |
| ESTHER : Schmidt_2014_Anal.Bioanal.Chem_406_5171 |
| PubMedSearch : Schmidt_2014_Anal.Bioanal.Chem_406_5171 |
| PubMedID: 24652148 |
| Title : Structural study of the complex stereoselectivity of human butyrylcholinesterase for the neurotoxic V-agents - Wandhammer_2011_J.Biol.Chem_286_16783 |
| Author(s) : Wandhammer M , Carletti E , van der Schans M , Gillon E , Nicolet Y , Masson P , Goeldner M , Noort D , Nachon F |
| Ref : Journal of Biological Chemistry , 286 :16783 , 2011 |
| Abstract : Wandhammer_2011_J.Biol.Chem_286_16783 |
| ESTHER : Wandhammer_2011_J.Biol.Chem_286_16783 |
| PubMedSearch : Wandhammer_2011_J.Biol.Chem_286_16783 |
| PubMedID: 21454498 |
| Gene_locus related to this paper: human-BCHE |
| Title : Unequal efficacy of pyridinium oximes in acute organophosphate poisoning - Antonijevic_2007_Clin.Med.Res_5_71 |
| Author(s) : Antonijevic B , Stojiljkovic MP |
| Ref : Clin Med Res , 5 :71 , 2007 |
| Abstract : Antonijevic_2007_Clin.Med.Res_5_71 |
| ESTHER : Antonijevic_2007_Clin.Med.Res_5_71 |
| PubMedSearch : Antonijevic_2007_Clin.Med.Res_5_71 |
| PubMedID: 17456837 |
| Title : Analysis of inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds with human and pig acetylcholinesterase - Aurbek_2006_Toxicology_224_91 |
| Author(s) : Aurbek N , Thiermann H , Szinicz L , Eyer P , Worek F |
| Ref : Toxicology , 224 :91 , 2006 |
| Abstract : Aurbek_2006_Toxicology_224_91 |
| ESTHER : Aurbek_2006_Toxicology_224_91 |
| PubMedSearch : Aurbek_2006_Toxicology_224_91 |
| PubMedID: 16720069 |