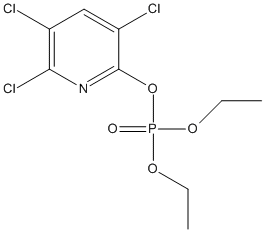
Chlorpyrifos-oxon
Results from the metabolism in vivo of chlorpyrifos (oxygen replaces the sulfur atom)
General
Type : Insecticide,Organophosphate
Chemical_Nomenclature : diethyl (3,5,6-trichloropyridin-2-yl) phosphate
Canonical SMILES : CCOP(=O)(OCC)OC1=NC(=C(C=C1Cl)Cl)Cl
InChI : InChI=1S\/C9H11Cl3NO4P\/c1-3-15-18(14,16-4-2)17-9-7(11)5-6(10)8(12)13-9\/h5H,3-4H2,1-2H3
InChIKey : OTMOUPHCTWPNSL-UHFFFAOYSA-N
Other name(s) : 3,5,6-trichloro-2-pyridyl diethyl phosphate,2,3,5-trichloro-6-diethoxyphosphoryloxy-pyridine,Dursbanoxon,Fospirate-ethyl,Chlorpyrifos oxon,Phospyrat ethyl,Chloropyrifos oxon,Dursban O-analog,Dursban oxygen analog,Chloropyrifos oxygen analog,CCRIS 7774,chlorpyriphos oxon,chlorpyryphos oxon,chlorpyryfos oxon
MW : 334.52
Formula : C9H11Cl3NO4P
CAS_number : 5598-15-2
PubChem : 21804
UniChem : OTMOUPHCTWPNSL-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Chlorpyrifos-oxon ligand of proteins in family: ACHE || ACPH_Peptidase_S9
Stucture :
Protein : human-APEH
References (11)
| Title : Chlorpyrifos oxon promotes tubulin aggregation via isopeptide cross-linking between diethoxyphospho-Lys and Glu or Asp: Implications for neurotoxicity - Schopfer_2018_J.Biol.Chem_293_13566 |
| Author(s) : Schopfer LM , Lockridge O |
| Ref : Journal of Biological Chemistry , 293 :13566 , 2018 |
| Abstract : Schopfer_2018_J.Biol.Chem_293_13566 |
| ESTHER : Schopfer_2018_J.Biol.Chem_293_13566 |
| PubMedSearch : Schopfer_2018_J.Biol.Chem_293_13566 |
| PubMedID: 30006344 |
| Title : Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents - Jiang_2010_Toxicol.Sci_115_183 |
| Author(s) : Jiang W , Duysen EG , Hansen H , Shlyakhtenko L , Schopfer LM , Lockridge O |
| Ref : Toxicol Sci , 115 :183 , 2010 |
| Abstract : Jiang_2010_Toxicol.Sci_115_183 |
| ESTHER : Jiang_2010_Toxicol.Sci_115_183 |
| PubMedSearch : Jiang_2010_Toxicol.Sci_115_183 |
| PubMedID: 20142434 |
| Title : Blood acylpeptide hydrolase activity is a sensitive marker for exposure to some organophosphate toxicants - Quistad_2005_Toxicol.Sci_86_291 |
| Author(s) : Quistad GB , Klintenberg R , Casida JE |
| Ref : Toxicol Sci , 86 :291 , 2005 |
| Abstract : Quistad_2005_Toxicol.Sci_86_291 |
| ESTHER : Quistad_2005_Toxicol.Sci_86_291 |
| PubMedSearch : Quistad_2005_Toxicol.Sci_86_291 |
| PubMedID: 15888665 |
| Gene_locus related to this paper: human-APEH |
| Title : Inhibition of carboxylesterases in SH-SY5Y human and NB41A3 mouse neuroblastoma cells by organophosphorus esters - Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| Author(s) : Ehrich M , Correll L |
| Ref : J Toxicol Environ Health , 53 :385 , 1998 |
| Abstract : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| ESTHER : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedSearch : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedID: 9515941 |
| Title : Rat brain acetylcholinesterase activity: developmental profile and maturational sensitivity to carbamate and organophosphorus inhibitors - Mortensen_1998_Toxicology_125_13 |
| Author(s) : Mortensen SR , Hooper MJ , Padilla S |
| Ref : Toxicology , 125 :13 , 1998 |
| Abstract : Mortensen_1998_Toxicology_125_13 |
| ESTHER : Mortensen_1998_Toxicology_125_13 |
| PubMedSearch : Mortensen_1998_Toxicology_125_13 |
| PubMedID: 9585096 |
| Title : Identification and isolation of two rat serum proteins with A-esterase activity toward paraoxon and chlorpyrifos-oxon - Pond_1996_Biochem.Pharmacol_52_363 |
| Author(s) : Pond AL , Coyne CP , Chambers HW , Chambers JE |
| Ref : Biochemical Pharmacology , 52 :363 , 1996 |
| Abstract : Pond_1996_Biochem.Pharmacol_52_363 |
| ESTHER : Pond_1996_Biochem.Pharmacol_52_363 |
| PubMedSearch : Pond_1996_Biochem.Pharmacol_52_363 |
| PubMedID: 8694862 |
| Title : Kinetic analysis of the in vitro inhibition, aging, and reactivation of brain acetylcholinesterase from rat and channel catfish by paraoxon and chlorpyrifos-oxon - Carr_1996_Toxicol.Appl.Pharmacol_139_365 |
| Author(s) : Carr RL , Chambers JE |
| Ref : Toxicology & Applied Pharmacology , 139 :365 , 1996 |
| Abstract : Carr_1996_Toxicol.Appl.Pharmacol_139_365 |
| ESTHER : Carr_1996_Toxicol.Appl.Pharmacol_139_365 |
| PubMedSearch : Carr_1996_Toxicol.Appl.Pharmacol_139_365 |
| PubMedID: 8806854 |
| Title : Inhibition and aging of channel catfish brain acetylcholinesterase following exposure to two phosphorothionate insecticides and their active metabolites - Carr_1995_J.Toxicol.Env.Health_45_325 |
| Author(s) : Carr RL , Straus DL , Chambers JE |
| Ref : Journal of Toxicology & Environmental Health , 45 :325 , 1995 |
| Abstract : Carr_1995_J.Toxicol.Env.Health_45_325 |
| ESTHER : Carr_1995_J.Toxicol.Env.Health_45_325 |
| PubMedSearch : Carr_1995_J.Toxicol.Env.Health_45_325 |
| PubMedID: 7541841 |
| Title : Inhibition patterns of brain acetylcholinesterase and hepatic and plasma aliesterases following exposures to three phosphorothionate insecticides and their oxons in rats - Chambers_1993_Fundam.Appl.Toxicol_21_111 |
| Author(s) : Chambers JE , Carr RL |
| Ref : Fundamental & Applied Toxicology , 21 :111 , 1993 |
| Abstract : Chambers_1993_Fundam.Appl.Toxicol_21_111 |
| ESTHER : Chambers_1993_Fundam.Appl.Toxicol_21_111 |
| PubMedSearch : Chambers_1993_Fundam.Appl.Toxicol_21_111 |
| PubMedID: 7689992 |
| Title : Serum paraoxonase status: a major factor in determining resistance to organophosphates - Li_1993_J.Toxicol.Env.Health_40_337 |
| Author(s) : Li WF , Costa LG , Furlong CE |
| Ref : Journal of Toxicology & Environmental Health , 40 :337 , 1993 |
| Abstract : Li_1993_J.Toxicol.Env.Health_40_337 |
| ESTHER : Li_1993_J.Toxicol.Env.Health_40_337 |
| PubMedSearch : Li_1993_J.Toxicol.Env.Health_40_337 |
| PubMedID: 7693961 |
| Title : Degradation by rat tissues in vitro of organophosphorus esters which inhibit cholinesterase - Pla_1989_Biochem.Pharmacol_38_1527 |
| Author(s) : Pla A , Johnson MK |
| Ref : Biochemical Pharmacology , 38 :1527 , 1989 |
| Abstract : Pla_1989_Biochem.Pharmacol_38_1527 |
| ESTHER : Pla_1989_Biochem.Pharmacol_38_1527 |
| PubMedSearch : Pla_1989_Biochem.Pharmacol_38_1527 |
| PubMedID: 2719724 |