Cocaine
Cocaine is a tropane alkaloid ester extracted from the leaves of plants including coca. It is a local anesthetic and vasoconstrictor and is clinically used for that purpose, particularly in the eye, ear, nose, and throat. It also has powerful central nervous system effects similar to the amphetamines and is a drug of abuse. Cocaine, like amphetamines, acts by multiple mechanisms on brain catecholaminergic neurons. Cocaine binds to the dopamine, serotonin, and norepinephrine transport proteins and inhibits the re-uptake of dopamine, serotonin, and norepinephrine into pre-synaptic neurons. This leads to an accumulation of the respective neurotransmitters in the synaptic cleft and may result in increased postsynaptic receptor activation. The mechanism of action through which cocaine exerts its local anesthetic effects is by binding to and blocking the voltage-gated sodium channels in the neuronal cell membrane. By stabilizing neuronal membranes, cocaine inhibits the initiation and conduction of nerve impulses and produces a reversible loss of sensation. (from PubChem) Cocaine is hydrolized by BChE and very efficiently by mutants of BChE
General
Type : Natural,Not A\/B H target,Drug,Alkaloid,Benzoate
Chemical_Nomenclature : 2beta-carbomethoxy-3beta-benzoyloxytropane hydrochloride
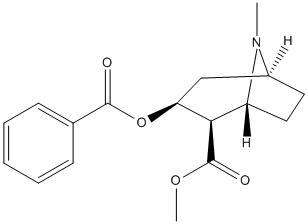
Canonical SMILES : CN1C2CCC1C(C(C2)OC(=O)C3=CC=CC=C3)C(=O)OC
InChI : InChI=1S\/C17H21NO4\/c1-18-12-8-9-13(18)15(17(20)21-2)14(10-12)22-16(19)11-6-4-3-5-7-11\/h3-7,12-15H,8-10H2,1-2H3\/t12-,13+,14-,15+\/m0\/s1
InChIKey : ZPUCINDJVBIVPJ-LJISPDSOSA-N
Other name(s) : (-)-Cocaine,3-beta-Hydroxy-, Methyl Ester, Benzoate,Methylbenzoylepgonine,Cocaine Free Base,Benzoylmethylecgonine,Crack,L-Cocain,beta-Cocain,D-cocaine
MW : 339.82
Formula : C17H21NO4.HCl
CAS_number : 53-21-4
PubChem : 446220
UniChem : ZPUCINDJVBIVPJ-LJISPDSOSA-N
IUPHAR : 2286
Wikipedia : Cocaine
Target
Families : Cocaine ligand of proteins in family: BCHE
Stucture : 3O9M Co-crystallization studies of full length recombinant BChE with cocaine offers insights into cocaine detoxification
Protein : human-BCHE
References (15)
| Title : Reward and Toxicity of Cocaine Metabolites Generated by Cocaine Hydrolase - Murthy_2015_Cell.Mol.Neurobiol_35_819 |
| Author(s) : Murthy V , Geng L , Gao Y , Zhang B , Miller JD , Reyes S , Brimijoin S |
| Ref : Cellular Molecular Neurobiology , 35 :819 , 2015 |
| Abstract : Murthy_2015_Cell.Mol.Neurobiol_35_819 |
| ESTHER : Murthy_2015_Cell.Mol.Neurobiol_35_819 |
| PubMedSearch : Murthy_2015_Cell.Mol.Neurobiol_35_819 |
| PubMedID: 25814464 |
| Title : Kinetic characterization of a cocaine hydrolase engineered from mouse butyrylcholinesterase - Chen_2015_Biochem.J_466_243 |
| Author(s) : Chen X , Huang X , Geng L , Xue L , Hou S , Zheng X , Brimijoin S , Zheng F , Zhan CG |
| Ref : Biochemical Journal , 466 :243 , 2015 |
| Abstract : Chen_2015_Biochem.J_466_243 |
| ESTHER : Chen_2015_Biochem.J_466_243 |
| PubMedSearch : Chen_2015_Biochem.J_466_243 |
| PubMedID: 25486543 |
| Gene_locus related to this paper: human-BCHE |
| Title : Rational design, preparation, and characterization of a therapeutic enzyme mutant with improved stability and function for cocaine detoxification - Fang_2014_ACS.Chem.Biol_9_1764 |
| Author(s) : Fang L , Chow KM , Hou S , Xue L , Chen X , Rodgers DW , Zheng F , Zhan CG |
| Ref : ACS Chemical Biology , 9 :1764 , 2014 |
| Abstract : Fang_2014_ACS.Chem.Biol_9_1764 |
| ESTHER : Fang_2014_ACS.Chem.Biol_9_1764 |
| PubMedSearch : Fang_2014_ACS.Chem.Biol_9_1764 |
| PubMedID: 24919140 |
| Gene_locus related to this paper: rhosm-cocE |
| Title : Preclinical studies on neurobehavioral and neuromuscular effects of cocaine hydrolase gene therapy in mice - Murthy_2014_J.Mol.Neurosci_53_409 |
| Author(s) : Murthy V , Gao Y , Geng L , LeBrasseur NK , White TA , Brimijoin S |
| Ref : Journal of Molecular Neuroscience , 53 :409 , 2014 |
| Abstract : Murthy_2014_J.Mol.Neurosci_53_409 |
| ESTHER : Murthy_2014_J.Mol.Neurosci_53_409 |
| PubMedSearch : Murthy_2014_J.Mol.Neurosci_53_409 |
| PubMedID: 24085526 |
| Title : The future potential for cocaine vaccines - Orson_2014_Expert.Opin.Biol.Ther_14_1271 |
| Author(s) : Orson FM , Wang R , Brimijoin S , Kinsey BM , Singh RA , Ramakrishnan M , Wang HY , Kosten TR |
| Ref : Expert Opin Biol Ther , 14 :1271 , 2014 |
| Abstract : Orson_2014_Expert.Opin.Biol.Ther_14_1271 |
| ESTHER : Orson_2014_Expert.Opin.Biol.Ther_14_1271 |
| PubMedSearch : Orson_2014_Expert.Opin.Biol.Ther_14_1271 |
| PubMedID: 24835496 |
| Title : A highly efficient cocaine-detoxifying enzyme obtained by computational design - Zheng_2014_Nat.Commun_5_3457 |
| Author(s) : Zheng F , Xue L , Hou S , Liu J , Zhan M , Yang W , Zhan CG |
| Ref : Nat Commun , 5 :3457 , 2014 |
| Abstract : Zheng_2014_Nat.Commun_5_3457 |
| ESTHER : Zheng_2014_Nat.Commun_5_3457 |
| PubMedSearch : Zheng_2014_Nat.Commun_5_3457 |
| PubMedID: 24643289 |
| Gene_locus related to this paper: human-BCHE |
| Title : Amino-acid mutations to extend the biological half-life of a therapeutically valuable mutant of human butyrylcholinesterase - Fang_2014_Chem.Biol.Interact_214C_18 |
| Author(s) : Fang L , Hou S , Xue L , Zheng F , Zhan CG |
| Ref : Chemico-Biological Interactions , 214C :18 , 2014 |
| Abstract : Fang_2014_Chem.Biol.Interact_214C_18 |
| ESTHER : Fang_2014_Chem.Biol.Interact_214C_18 |
| PubMedSearch : Fang_2014_Chem.Biol.Interact_214C_18 |
| PubMedID: 24582612 |
| Gene_locus related to this paper: human-BCHE |
| Title : Plants as a source of butyrylcholinesterase variants designed for enhanced cocaine hydrolase activity - Larrimore_2013_Chem.Biol.Interact_203_217 |
| Author(s) : Larrimore KE , Barcus M , Kannan L , Gao Y , Zhan CG , Brimijoin S , Mor TS |
| Ref : Chemico-Biological Interactions , 203 :217 , 2013 |
| Abstract : Larrimore_2013_Chem.Biol.Interact_203_217 |
| ESTHER : Larrimore_2013_Chem.Biol.Interact_203_217 |
| PubMedSearch : Larrimore_2013_Chem.Biol.Interact_203_217 |
| PubMedID: 23000451 |
| Title : Catalytic activities of a cocaine hydrolase engineered from human butyrylcholinesterase against (+)- and (-)-cocaine - Xue_2013_Chem.Biol.Interact_203_57 |
| Author(s) : Xue L , Hou S , Yang W , Fang L , Zheng F , Zhan CG |
| Ref : Chemico-Biological Interactions , 203 :57 , 2013 |
| Abstract : Xue_2013_Chem.Biol.Interact_203_57 |
| ESTHER : Xue_2013_Chem.Biol.Interact_203_57 |
| PubMedSearch : Xue_2013_Chem.Biol.Interact_203_57 |
| PubMedID: 22917637 |
| Title : Cocaine metabolism accelerated by a re-engineered human butyrylcholinesterase - Sun_2002_J.Pharmacol.Exp.Ther_302_710 |
| Author(s) : Sun H , Shen ML , Pang YP , Lockridge O , Brimijoin S |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 302 :710 , 2002 |
| Abstract : Sun_2002_J.Pharmacol.Exp.Ther_302_710 |
| ESTHER : Sun_2002_J.Pharmacol.Exp.Ther_302_710 |
| PubMedSearch : Sun_2002_J.Pharmacol.Exp.Ther_302_710 |
| PubMedID: 12130735 |
| Gene_locus related to this paper: human-BCHE |
| Title : Re-engineering butyrylcholinesterase as a cocaine hydrolase - Sun_2002_Mol.Pharmacol_62_220 |
| Author(s) : Sun H , Pang YP , Lockridge O , Brimijoin S |
| Ref : Molecular Pharmacology , 62 :220 , 2002 |
| Abstract : Sun_2002_Mol.Pharmacol_62_220 |
| ESTHER : Sun_2002_Mol.Pharmacol_62_220 |
| PubMedSearch : Sun_2002_Mol.Pharmacol_62_220 |
| PubMedID: 12130672 |
| Gene_locus related to this paper: human-BCHE |
| Title : Predicted Michaelis-Menten complexes of cocaine-butyrylcholinesterase. Engineering effective butyrylcholinesterase mutants for cocaine detoxication. - Sun_2001_J.Biol.Chem_276_9330 |
| Author(s) : Sun H , El Yazal J , Lockridge O , Schopfer LM , Brimijoin S , Pang YP |
| Ref : Journal of Biological Chemistry , 276 :9330 , 2001 |
| Abstract : Sun_2001_J.Biol.Chem_276_9330 |
| ESTHER : Sun_2001_J.Biol.Chem_276_9330 |
| PubMedSearch : Sun_2001_J.Biol.Chem_276_9330 |
| PubMedID: 11104759 |
| Title : Stereoselective inhibition of human butyrylcholinesterase by phosphonothiolate analogs of (+)- and (-)-cocaine - Berkman_1997_Biochem.Pharmacol_54_1261 |
| Author(s) : Berkman CE , Underiner GE , Cashman JR |
| Ref : Biochemical Pharmacology , 54 :1261 , 1997 |
| Abstract : Berkman_1997_Biochem.Pharmacol_54_1261 |
| ESTHER : Berkman_1997_Biochem.Pharmacol_54_1261 |
| PubMedSearch : Berkman_1997_Biochem.Pharmacol_54_1261 |
| PubMedID: 9416977 |
| Title : Overlapping drug interaction sites of human butyrylcholinesterase dissected by site-directed mutagenesis - Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| Author(s) : Loewenstein-Lichtenstein Y , Glick D , Gluzman N , Sternfeld M , Zakut H , Soreq H |
| Ref : Molecular Pharmacology , 50 :1423 , 1996 |
| Abstract : Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| ESTHER : Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| PubMedSearch : Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| PubMedID: 8967962 |
| Gene_locus related to this paper: human-BCHE |
| Title : Hydrolysis of cocaine in human plasma by cholinesterase - |
| Author(s) : Stewart DJ , Inaba T , Tang BK , Kalow W |
| Ref : Life Sciences , 20 :1557 , 1977 |
| PubMedID: 17804 |