CyC-17
General
Type : Organophosphate,Cyclic OP,Lipase inhibitor
Chemical_Nomenclature :
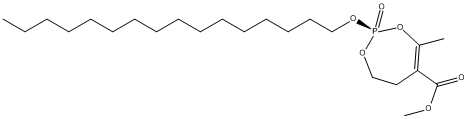
Canonical SMILES : CCCCCCCCCCCCCCCCO[P@]1(=O)OCCC(=C(O1)C)C(=O)C
InChI : InChI=1S\/C23H43O6P\/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-19-27-30(25)28-20-18-22(21(2)29-30)23(24)26-3\/h4-20H2,1-3H3\/t30-\/m1\/s1
InChIKey : UQUOYRVMTVUYSM-SSEXGKCCSA-N
Other name(s) : CyC17,ZINC000299869404,ZINC299869404
MW : 446.28
Formula : C23H43O6P
CAS_number :
PubChem :
UniChem : UQUOYRVMTVUYSM-SSEXGKCCSA-N
IUPHAR :
Wikipedia :
Target
Families : CyC-17 ligand of proteins in family: A85-Mycolyl-transferase || Hormone-sensitive_lipase_like || Cutinase || Carbon-carbon_bond_hydrolase || Thioesterase
Stucture :
Protein : myctu-a85c || ratno-hslip || myctu-cutas1 || myctu-Rv3569c || myctu-a85a || myctu-yt28
References (6)
| Title : Identification of cell wall synthesis inhibitors active against Mycobacterium tuberculosis by competitive activity-based protein profiling - Li_2022_Cell.Chem.Biol_29_883 |
| Author(s) : Li M , Patel HV , Cognetta AB, 3rd , Smith TC, 2nd , Mallick I , Cavalier JF , Previti ML , Canaan S , Aldridge BB , Cravatt BF , Seeliger JC |
| Ref : Cell Chemical Biology , 29 :883 , 2022 |
| Abstract : Li_2022_Cell.Chem.Biol_29_883 |
| ESTHER : Li_2022_Cell.Chem.Biol_29_883 |
| PubMedSearch : Li_2022_Cell.Chem.Biol_29_883 |
| PubMedID: 34599873 |
| Title : Cyclophostin and Cyclipostins analogues, new promising molecules to treat mycobacterial-related diseases - Nguyen_2018_Int.J.Antimicrob.Agents_51_651 |
| Author(s) : Nguyen PC , Madani A , Santucci P , Martin BP , Paudel RR , Delattre S , Herrmann JL , Spilling CD , Kremer L , Canaan S , Cavalier JF |
| Ref : Int J Antimicrob Agents , 51 :651 , 2018 |
| Abstract : Nguyen_2018_Int.J.Antimicrob.Agents_51_651 |
| ESTHER : Nguyen_2018_Int.J.Antimicrob.Agents_51_651 |
| PubMedSearch : Nguyen_2018_Int.J.Antimicrob.Agents_51_651 |
| PubMedID: 29241819 |
| Title : Biochemical and Structural Characterization of TesA, a Major Thioesterase Required for Outer-Envelope Lipid Biosynthesis in Mycobacterium tuberculosis - Nguyen_2018_J.Mol.Biol_430_5120 |
| Author(s) : Nguyen PC , Nguyen VS , Martin BP , Fourquet P , Camoin L , Spilling CD , Cavalier JF , Cambillau C , Canaan S |
| Ref : Journal of Molecular Biology , 430 :5120 , 2018 |
| Abstract : Nguyen_2018_J.Mol.Biol_430_5120 |
| ESTHER : Nguyen_2018_J.Mol.Biol_430_5120 |
| PubMedSearch : Nguyen_2018_J.Mol.Biol_430_5120 |
| PubMedID: 30292819 |
| Gene_locus related to this paper: myctu-yt28 |
| Title : Cyclipostins and cyclophostin analogs inhibit the antigen 85C from Mycobacterium tuberculosis both in vitro and in vivo - Viljoen_2018_J.Biol.Chem_293_2755 |
| Author(s) : Viljoen A , Richard M , Nguyen PC , Fourquet P , Camoin L , Paudal RR , Gnawali GR , Spilling CD , Cavalier JF , Canaan S , Blaise M , Kremer L |
| Ref : Journal of Biological Chemistry , 293 :2755 , 2018 |
| Abstract : Viljoen_2018_J.Biol.Chem_293_2755 |
| ESTHER : Viljoen_2018_J.Biol.Chem_293_2755 |
| PubMedSearch : Viljoen_2018_J.Biol.Chem_293_2755 |
| PubMedID: 29301937 |
| Gene_locus related to this paper: myctu-a85c |
| Title : Cyclipostins and Cyclophostin analogs as promising compounds in the fight against tuberculosis - Nguyen_2017_Sci.Rep_7_11751 |
| Author(s) : Nguyen PC , Delorme V , Benarouche A , Martin BP , Paudel R , Gnawali GR , Madani A , Puppo R , Landry V , Kremer L , Brodin P , Spilling CD , Cavalier JF , Canaan S |
| Ref : Sci Rep , 7 :11751 , 2017 |
| Abstract : Nguyen_2017_Sci.Rep_7_11751 |
| ESTHER : Nguyen_2017_Sci.Rep_7_11751 |
| PubMedSearch : Nguyen_2017_Sci.Rep_7_11751 |
| PubMedID: 28924204 |
| Gene_locus related to this paper: mycle-ML2603 , myctu-a85a , myctu-a85c , myctu-Rv1399c , myctu-Rv2970c , myctu-Rv3569c , myctu-yt28 |
| Title : Rat hormone sensitive lipase inhibition by cyclipostins and their analogs - Vasilieva_2015_Bioorg.Med.Chem_23_944 |
| Author(s) : Vasilieva E , Dutta S , Malla RK , Martin BP , Spilling CD , Dupureur CM |
| Ref : Bioorganic & Medicinal Chemistry , 23 :944 , 2015 |
| Abstract : Vasilieva_2015_Bioorg.Med.Chem_23_944 |
| ESTHER : Vasilieva_2015_Bioorg.Med.Chem_23_944 |
| PubMedSearch : Vasilieva_2015_Bioorg.Med.Chem_23_944 |
| PubMedID: 25678014 |
| Gene_locus related to this paper: ratno-hslip |