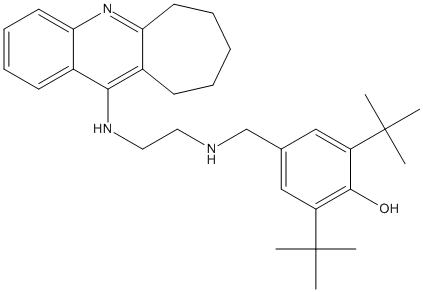
Di-tert-butyl-4-Amino-2,3-cycloheptaquinoline-8c
IC50(AChE) = 1.90 +/- 0.16 microM, IC50(BChE) = 0.084 +/- 0.008 microM, and 13.6 +/- 1.2% propidium displacement at 20 microM
General
Type : Quinoline,Multitarget,Antioxidant,tert-Butyl
Chemical_Nomenclature : 2,6-Di-tert-butyl-4-{[2-(7,8,9,10-tetrahydro-6H-cyclohepta[b]quinolin-11-ylamino)-ethylamino]-methyl}-phenol
Canonical SMILES : C1=CC=C2C(=C1)N=C4C(=C2NCCNCC3=CC(=C(C(=C3)C(C)(C)C)O)C(C)(C)C)CCCCC4
InChI : InChI=1S\/C31H43N3O\/c1-30(2,3)24-18-21(19-25(29(24)35)31(4,5)6)20-32-16-17-33-28-22-12-8-7-9-14-26(22)34-27-15-11-10-13-23(27)28\/h10-11,13,15,18-19,32,35H,7-9,12,14,16-17,20H2,1-6H3,(H,33,34)
InChIKey : ZQEJEBOCGKSUKW-UHFFFAOYSA-N
Other name(s) :
MW : 473.70
Formula : C31H43N3O
CAS_number :
PubChem :
UniChem : ZQEJEBOCGKSUKW-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Di-tert-butyl-4-Amino-2,3-cycloheptaquinoline-8c ligand of proteins in family: BCHE
Stucture :
Protein :
References (1)
| Title : New Multifunctional Agents Based on Conjugates of 4-Amino-2,3-polymethylenequinoline and Butylated Hydroxytoluene for Alzheimer's Disease Treatment - Makhaeva_2020_Molecules_25_5891 |
| Author(s) : Makhaeva GF , Kovaleva NV , Rudakova EV , Boltneva NP , Lushchekina SV , Faingold, II , Poletaeva DA , Soldatova YV , Kotelnikova RA , Serkov IV , Ustinov AK , Proshin AN , Radchenko EV , Palyulin VA , Richardson RJ |
| Ref : Molecules , 25 :5891 , 2020 |
| Abstract : Makhaeva_2020_Molecules_25_5891 |
| ESTHER : Makhaeva_2020_Molecules_25_5891 |
| PubMedSearch : Makhaeva_2020_Molecules_25_5891 |
| PubMedID: 33322783 |