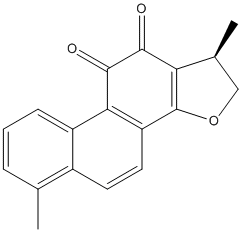
Dihydrotanshinone-I
Mycotoxin purified from culture of fungus Aspergillus terreus 23-1 growing on rice. TerritremB spans both catalytic and peripheral sites whereas dihydrotanshinone I binds only to the peripheral site (Cheung et al. 2013)
General
Type : Natural,Benzofuran
Chemical_Nomenclature : (1R)-1,6-dimethyl-1,2-dihydronaphtho[1,2-g][1]benzofuran-10,11-dione
Canonical SMILES : CC1COC2=C1C(=O)C(=O)C3=C2C=CC4=C3C=CC=C4C
InChI : InChI=1S\/C18H14O3\/c1-9-4-3-5-12-11(9)6-7-13-15(12)17(20)16(19)14-10(2)8-21-18(13)14\/h3-7,10H,8H2,1-2H3\/t10-\/m0\/s1
InChIKey : HARGZZNYNSYSGJ-JTQLQIEISA-N
Other name(s) : 15,16-dihydrotanshinone I,Tanshinone I, dihydro-
MW : 278.30
Formula : C18H14O3
CAS_number : 87205-99-0
PubChem : 11425923
UniChem : HARGZZNYNSYSGJ-JTQLQIEISA-N
IUPHAR :
Wikipedia :
Target
Families : Dihydrotanshinone-I ligand of proteins in family: ACHE || Carb_B_Chordata
Stucture : 4M0E Structure of human acetylcholinesterase in complex with dihydrotanshinone I
Protein : human-ACHE
References (5)
| Title : Acetylcholinesterase complexes with the natural product inhibitors dihydrotanshinone I and territrem B: binding site assignment from inhibitor competition and validation through crystal structure determination - Cheung_2014_J.Mol.Neurosci_53_506 |
| Author(s) : Cheung J , Beri V , Shiomi K , Rosenberry TL |
| Ref : Journal of Molecular Neuroscience , 53 :506 , 2014 |
| Abstract : Cheung_2014_J.Mol.Neurosci_53_506 |
| ESTHER : Cheung_2014_J.Mol.Neurosci_53_506 |
| PubMedSearch : Cheung_2014_J.Mol.Neurosci_53_506 |
| PubMedID: 24573600 |
| Title : Structures of Human Acetylcholinesterase Bound to Dihydrotanshinone I and Territrem B Show Peripheral Site Flexibility - Cheung_2013_ACS.Med.Chem.Lett_4_1091 |
| Author(s) : Cheung J , Gary EN , Shiomi K , Rosenberry TL |
| Ref : ACS Med Chem Lett , 4 :1091 , 2013 |
| Abstract : Cheung_2013_ACS.Med.Chem.Lett_4_1091 |
| ESTHER : Cheung_2013_ACS.Med.Chem.Lett_4_1091 |
| PubMedSearch : Cheung_2013_ACS.Med.Chem.Lett_4_1091 |
| PubMedID: 24900610 |
| Gene_locus related to this paper: human-ACHE |
| Title : The natural product dihydrotanshinone I provides a prototype for uncharged inhibitors that bind specifically to the acetylcholinesterase peripheral site with nanomolar affinity - Beri_2013_Biochemistry_52_7486 |
| Author(s) : Beri V , Wildman SA , Shiomi K , Al-Rashid ZF , Cheung J , Rosenberry TL |
| Ref : Biochemistry , 52 :7486 , 2013 |
| Abstract : Beri_2013_Biochemistry_52_7486 |
| ESTHER : Beri_2013_Biochemistry_52_7486 |
| PubMedSearch : Beri_2013_Biochemistry_52_7486 |
| PubMedID: 24040835 |
| Title : Modulation of esterified drug metabolism by tanshinones from Salvia miltiorrhiza (Danshen) - Hatfield_2013_J.Nat.Prod_76_36 |
| Author(s) : Hatfield MJ , Tsurkan LG , Hyatt JL , Edwards CC , Lemoff A , Jeffries C , Yan B , Potter PM |
| Ref : Journal of Natural Products , 76 :36 , 2013 |
| Abstract : Hatfield_2013_J.Nat.Prod_76_36 |
| ESTHER : Hatfield_2013_J.Nat.Prod_76_36 |
| PubMedSearch : Hatfield_2013_J.Nat.Prod_76_36 |
| PubMedID: 23286284 |
| Title : Interaction study of two diterpenes, cryptotanshinone and dihydrotanshinone, to human acetylcholinesterase and butyrylcholinesterase by molecular docking and kinetic analysis - Wong_2010_Chem.Biol.Interact_187_335 |
| Author(s) : Wong KK , Ngo JC , Liu S , Lin HQ , Hu C , Shaw PC , Wan DC |
| Ref : Chemico-Biological Interactions , 187 :335 , 2010 |
| Abstract : Wong_2010_Chem.Biol.Interact_187_335 |
| ESTHER : Wong_2010_Chem.Biol.Interact_187_335 |
| PubMedSearch : Wong_2010_Chem.Biol.Interact_187_335 |
| PubMedID: 20350537 |