Ebelactone-A
General
Type : Oxooxetan,Natural
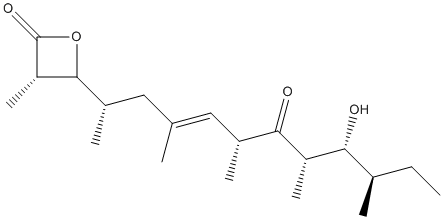
Chemical_Nomenclature : (3S,4S)-4-[(E,2S,6R,8S,9R,10R)-9-hydroxy-4,6,8,10-tetramethyl-7-oxododec-4-en-2-yl]-3-methyloxetan-2-one
Canonical SMILES : CCC(C)C(C(C)C(=O)C(C)C=C(C)CC(C)C1C(C(=O)O1)C)O
InChI : InChI=1S\/C20H34O4\/c1-8-12(3)17(21)15(6)18(22)13(4)9-11(2)10-14(5)19-16(7)20(23)24-19\/h9,12-17,19,21H,8,10H2,1-7H3\/b11-9+\/t12-,13-,14+,15+,16+,17-,19+\/m1\/s1
InChIKey : WOISDAHQBUYEAF-QIQXJRRPSA-N
Other name(s) : Ebelactone a,Ebelactone A microbial,NSC 335650,2-Oxetanone, 4-(8-hydroxy-1,3,5,7,9-pentamethyl-6-oxo-3-undecenyl)-3-methyl-, (3S-(3-alpha,4-beta(1R*,3E,5S*,7R*,8S*,9S*)))-,76808-16-7,3,11-Dihydroxy-2,4,6,8,10,12-hexamethyl-9-oxo-6-tetradecenoic acid 1,3-lactone
MW : 338.48
Formula : C20H34O4
CAS_number :
PubChem : 6436820
UniChem : WOISDAHQBUYEAF-QIQXJRRPSA-N
IUPHAR :
Wikipedia :
Target
Families : Ebelactone-A ligand of proteins in family: Homoserine_transacetylase || Pancreatic_lipase || ACPH_Peptidase_S9
Stucture :
Protein :
References (6)
| Title : Biosynthesis of ebelactone A: isotopic tracer, advanced precursor and genetic studies reveal a thioesterase-independent cyclization to give a polyketide beta-lactone - Wyatt_2013_J.Antibiot.(Tokyo)_66_421 |
| Author(s) : Wyatt MA , Ahilan Y , Argyropoulos P , Boddy CN , Magarvey NA , Harrison PH |
| Ref : J Antibiot (Tokyo) , 66 :421 , 2013 |
| Abstract : Wyatt_2013_J.Antibiot.(Tokyo)_66_421 |
| ESTHER : Wyatt_2013_J.Antibiot.(Tokyo)_66_421 |
| PubMedSearch : Wyatt_2013_J.Antibiot.(Tokyo)_66_421 |
| PubMedID: 23801186 |
| Title : beta-Lactone natural products and derivatives inactivate homoserine transacetylase, a target for antimicrobial agents - De Pascale_2011_J.Antibiot.(Tokyo)_64_483 |
| Author(s) : De Pascale G , Nazi I , Harrison PH , Wright GD |
| Ref : J Antibiot (Tokyo) , 64 :483 , 2011 |
| Abstract : De Pascale_2011_J.Antibiot.(Tokyo)_64_483 |
| ESTHER : De Pascale_2011_J.Antibiot.(Tokyo)_64_483 |
| PubMedSearch : De Pascale_2011_J.Antibiot.(Tokyo)_64_483 |
| PubMedID: 21522158 |
| Gene_locus related to this paper: haein-metx |
| Title : Effects of ebelactone B, a lipase inhibitor, on intestinal fat absorption in the rat - Nonaka_1996_J.Enzyme.Inhib_10_57 |
| Author(s) : Nonaka Y , Ohtaki H , Ohtsuka E , Kocha T , Fukuda T , Takeuchi T , Aoyagi T |
| Ref : J Enzyme Inhib , 10 :57 , 1996 |
| Abstract : Nonaka_1996_J.Enzyme.Inhib_10_57 |
| ESTHER : Nonaka_1996_J.Enzyme.Inhib_10_57 |
| PubMedSearch : Nonaka_1996_J.Enzyme.Inhib_10_57 |
| PubMedID: 8835930 |
| Title : Human acylpeptide hydrolase. Studies on its thiol groups and mechanism of action - Scaloni_1994_J.Biol.Chem_269_15076 |
| Author(s) : Scaloni A , Barra D , Jones WM , Manning JM |
| Ref : Journal of Biological Chemistry , 269 :15076 , 1994 |
| Abstract : Scaloni_1994_J.Biol.Chem_269_15076 |
| ESTHER : Scaloni_1994_J.Biol.Chem_269_15076 |
| PubMedSearch : Scaloni_1994_J.Biol.Chem_269_15076 |
| PubMedID: 8195144 |
| Title : Acylpeptide hydrolase: inhibitors and some active site residues of the human enzyme - Scaloni_1992_J.Biol.Chem_267_3811 |
| Author(s) : Scaloni A , Jones WM , Barra D , Pospischil M , Sassa S , Popowicz AM , Manning LR , Schneewind O , Manning JM |
| Ref : Journal of Biological Chemistry , 267 :3811 , 1992 |
| Abstract : Scaloni_1992_J.Biol.Chem_267_3811 |
| ESTHER : Scaloni_1992_J.Biol.Chem_267_3811 |
| PubMedSearch : Scaloni_1992_J.Biol.Chem_267_3811 |
| PubMedID: 1740429 |