Galanthaminium
IC50 1.4 +/- 0.2 (10-7 M) , more potent than Galanthamine (Mary et al 1998).Structure of torca-ACHE inhibited Greenblatt_2004 1W6R
General
Type : Derivative of Galanthamine,Alkaloid,Natural_modified,Benzazepin,Azepine
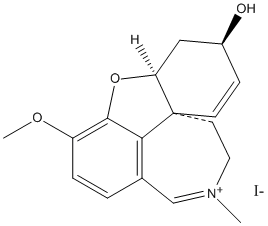
Chemical_Nomenclature : (1S,14R)-9-methoxy-4-methyl-11-oxa-4-azoniatetracyclo[8.6.1.01,12.06,17]heptadeca-4,6(17),7,9,15-pentaen-14-ol
Canonical SMILES : C[N+]1=CC2=C3C(=C(C=C2)OC)OC4C3(CC1)C=CC(C4)O
InChI : InChI=1S\/C17H20NO3\/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17\/h3-6,10,12,14,19H,7-9H2,1-2H3\/q+1\/t12-,14?,17-\/m0\/s1
InChIKey : RKYCDHLWOSUDFI-XTIDWKMFSA-N
Other name(s) : (-)-9-Dehydrogalanthaminium,CID11199388,Galanthamine derivative 5
MW : 286.35
Formula : C17H20N03
CAS_number :
PubChem : 11165169
UniChem : RKYCDHLWOSUDFI-XTIDWKMFSA-N
IUPHAR :
Wikipedia :
Target
References (3)
| Title : The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: implications for structure-based drug design - Greenblatt_2004_J.Am.Chem.Soc_126_15405 |
| Author(s) : Greenblatt HM , Guillou C , Guenard D , Argaman A , Botti SA , Badet B , Thal C , Silman I , Sussman JL |
| Ref : Journal of the American Chemical Society , 126 :15405 , 2004 |
| Abstract : Greenblatt_2004_J.Am.Chem.Soc_126_15405 |
| ESTHER : Greenblatt_2004_J.Am.Chem.Soc_126_15405 |
| PubMedSearch : Greenblatt_2004_J.Am.Chem.Soc_126_15405 |
| PubMedID: 15563167 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Combined treatment with galanthaminium bromide, a new cholinesterase inhibitor, and RS 67333, a partial agonist of 5-HT4 receptors, enhances place and object recognition in young adult and old rats - Lamirault_2003_Prog.Neuropsychopharmacol.Biol.Psychiatry_27_185 |
| Author(s) : Lamirault L , Guillou C , Thal C , Simon H |
| Ref : Prog Neuropsychopharmacol Biological Psychiatry , 27 :185 , 2003 |
| Abstract : Lamirault_2003_Prog.Neuropsychopharmacol.Biol.Psychiatry_27_185 |
| ESTHER : Lamirault_2003_Prog.Neuropsychopharmacol.Biol.Psychiatry_27_185 |
| PubMedSearch : Lamirault_2003_Prog.Neuropsychopharmacol.Biol.Psychiatry_27_185 |
| PubMedID: 12551743 |
| Title : Potent acetylcholinesterase inhibitors: design, synthesis, and structure-activity relationships of bis-interacting ligands in the galanthamine series - Mary_1998_Bioorg.Med.Chem_6_1835 |
| Author(s) : Mary A , Renko DZ , Guillou C , Thal C |
| Ref : Bioorganic & Medicinal Chemistry , 6 :1835 , 1998 |
| Abstract : Mary_1998_Bioorg.Med.Chem_6_1835 |
| ESTHER : Mary_1998_Bioorg.Med.Chem_6_1835 |
| PubMedSearch : Mary_1998_Bioorg.Med.Chem_6_1835 |
| PubMedID: 9839013 |