Glycidyl-Palmitoleate
General
Type : Analogue of substrate,Epoxide,Oleate
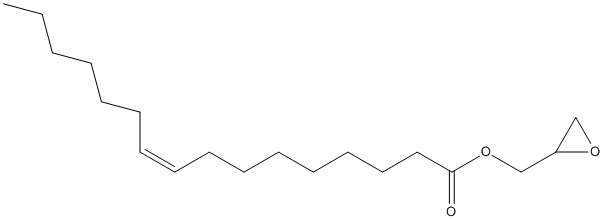
Chemical_Nomenclature : oxiran-2-ylmethyl (Z)-hexadec-9-enoate
Canonical SMILES : CCCCCCC=CCCCCCCCC(=O)OCC1CO1
InChI : InChI=1S\/C19H34O3\/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-19(20)22-17-18-16-21-18\/h7-8,18H,2-6,9-17H2,1H3\/b8-7-
InChIKey : FWEHVPCBXKCYHE-FPLPWBNLSA-N
Other name(s) : UCM710,(+\/-)-oxiran-2-ylmethyl (9Z)-hexadec-9-enoate,oxiran-2-ylmethyl (9z)-hexadec-9-enoate,CHEMBL230968,Glycidyl Palmitoleate,SCHEMBL4979121
MW : 310.5
Formula : C19H34O3
CAS_number : 213738-77-3
PubChem : 23624909
UniChem : FWEHVPCBXKCYHE-FPLPWBNLSA-N
IUPHAR :
Wikipedia :
Target
Families : Glycidyl-Palmitoleate ligand of proteins in family: ABHD6-Lip
Stucture :
Protein : human-ABHD6
References (1)
| Title : Dual inhibition of alpha\/beta-hydrolase domain 6 and fatty acid amide hydrolase increases endocannabinoid levels in neurons - Marrs_2011_J.Biol.Chem_286_28723 |
| Author(s) : Marrs WR , Horne EA , Ortega-Gutierrez S , Cisneros JA , Xu C , Lin YH , Muccioli GG , Lopez-Rodriguez ML , Stella N |
| Ref : Journal of Biological Chemistry , 286 :28723 , 2011 |
| Abstract : Marrs_2011_J.Biol.Chem_286_28723 |
| ESTHER : Marrs_2011_J.Biol.Chem_286_28723 |
| PubMedSearch : Marrs_2011_J.Biol.Chem_286_28723 |
| PubMedID: 21665953 |
| Gene_locus related to this paper: human-ABHD6 |