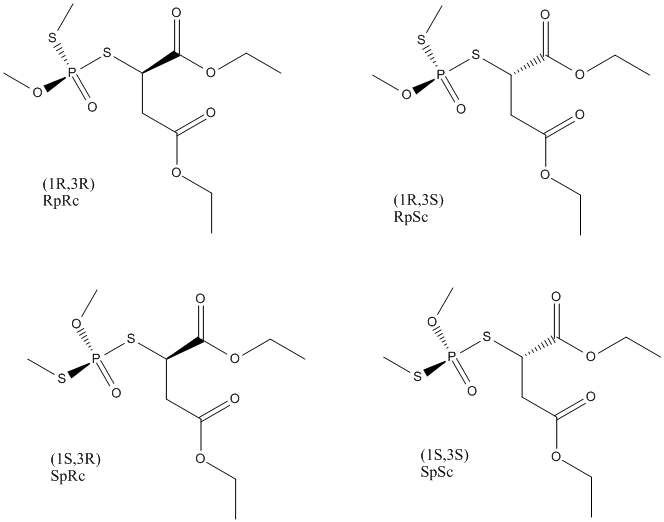
Isomalathion
Impurity in malathion extremely toxic for mammals || Results from isomerization of phosphorothionate to phosphorothiolate. It gains a chiral center and has four stereoisomers which show stereoselective action against cholinesterase || position of sulfure and oxygene inverse of malathion
General
Type : Organophosphate,Sulfur Compound,Organothiophosphate
Chemical_Nomenclature : diethyl 2-(methoxy-methylsulfanyl-phosphoryl)sulfanylbutanedioate
Canonical SMILES : CCOC(=O)CC(C(=O)OCC)SP(=O)(OC)SC
InChI : InChI=1S\/C10H19O6PS2\/c1-5-15-9(11)7-8(10(12)16-6-2)19-17(13,14-3)18-4\/h8H,5-7H2,1-4H3
InChIKey : LPQDGVLVYVULMX-UHFFFAOYSA-N
Other name(s) : S-Methylmalathion,CCRIS 5620,8063HC
MW : 330.360
Formula : C10H19O6PS2
CAS_number : 3344-12-5
PubChem : 91529
UniChem : LPQDGVLVYVULMX-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Isomalathion
Target
References (8)
| Title : Kinetic Evidence for Different Mechanisms of Acetylcholinesterase Inhibition by (1R)- and (1S)-Stereoisomers of Isomalathion - Jianmongkol_1999_Toxicol.Appl.Pharmacol_155_43 |
| Author(s) : Jianmongkol S , Marable BR , Berkman CE , Talley TT , Thompson CM , Richardson RJ |
| Ref : Toxicol Appl Pharmacol , 155 :43 , 1999 |
| Abstract : Jianmongkol_1999_Toxicol.Appl.Pharmacol_155_43 |
| ESTHER : Jianmongkol_1999_Toxicol.Appl.Pharmacol_155_43 |
| PubMedSearch : Jianmongkol_1999_Toxicol.Appl.Pharmacol_155_43 |
| PubMedID: 10036217 |
| Title : Synthesis and 31P chemical shift identification of tripeptide active site models that represent human serum acetylcholinesterase covalently modified at serine by certain organophosphates - Thompson_1996_Chem.Res.Toxicol_9_1325 |
| Author(s) : Thompson CM , Suarez AI , Rodriguez OP |
| Ref : Chemical Research in Toxicology , 9 :1325 , 1996 |
| Abstract : Thompson_1996_Chem.Res.Toxicol_9_1325 |
| ESTHER : Thompson_1996_Chem.Res.Toxicol_9_1325 |
| PubMedSearch : Thompson_1996_Chem.Res.Toxicol_9_1325 |
| PubMedID: 8951236 |
| Title : Synthesis, absolute configuration, and analysis of malathion, malaoxon, and isomalathion enantiomers [published erratum appears in Chem Res Toxicol 1994 Mar-Apr\;7(2):275] - Berkman_1993_Chem.Res.Toxicol_6_718 |
| Author(s) : Berkman CE , Thompson CM , Perrin SR |
| Ref : Chemical Research in Toxicology , 6 :718 , 1993 |
| Abstract : Berkman_1993_Chem.Res.Toxicol_6_718 |
| ESTHER : Berkman_1993_Chem.Res.Toxicol_6_718 |
| PubMedSearch : Berkman_1993_Chem.Res.Toxicol_6_718 |
| PubMedID: 8292751 |
| Title : An enzyme test for determining isomalathion impurities in water-dispersible powders of malathion - Reiner_1986_Bull.World.Health.Organ_64_397 |
| Author(s) : Reiner E , Radic Z |
| Ref : Bulletin of the World Health Organization , 64 :397 , 1986 |
| Abstract : Reiner_1986_Bull.World.Health.Organ_64_397 |
| ESTHER : Reiner_1986_Bull.World.Health.Organ_64_397 |
| PubMedSearch : Reiner_1986_Bull.World.Health.Organ_64_397 |
| PubMedID: 3490319 |
| Title : Mutagenic and alkylating activities of organophosphate impurities of commercial malathion - Imamura_1985_Mutat.Res_155_1 |
| Author(s) : Imamura T , Talcott RE |
| Ref : Mutat Res , 155 :1 , 1985 |
| Abstract : Imamura_1985_Mutat.Res_155_1 |
| ESTHER : Imamura_1985_Mutat.Res_155_1 |
| PubMedSearch : Imamura_1985_Mutat.Res_155_1 |
| PubMedID: 3881662 |
| Title : Toxicological properties of trialkyl phosphorothioate and dialkyl alkyl- and arylphosphonothioate esters - Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| Author(s) : Fukuto TR |
| Ref : J Environ Sci Health B , 18 :89 , 1983 |
| Abstract : Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| ESTHER : Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| PubMedSearch : Fukuto_1983_J.Environ.Sci.Health.B_18_89 |
| PubMedID: 6833715 |
| Title : The toxicological properties of impurities in malathion - Aldridge_1979_Arch.Toxicol_42_95 |
| Author(s) : Aldridge WN , Miles JW , Mount DL , Verschoyle RD |
| Ref : Archives of Toxicology , 42 :95 , 1979 |
| Abstract : Aldridge_1979_Arch.Toxicol_42_95 |
| ESTHER : Aldridge_1979_Arch.Toxicol_42_95 |
| PubMedSearch : Aldridge_1979_Arch.Toxicol_42_95 |
| PubMedID: 755464 |
| Title : Preparation & assay of isomalathion - |
| Author(s) : Ramakrishna N , Ramachandran BV |
| Ref : Indian J Biochem Biophys , 15 :80 , 1978 |
| PubMedID: 721118 |