JCP276
Low selectivity. Inhibits multiple mycobacterium serine hydrolases
General
Type : Coumarin,Urea derivative
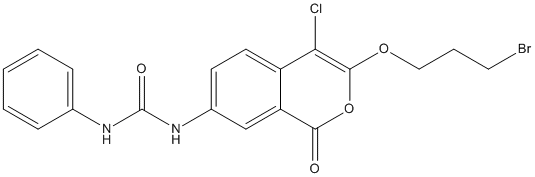
Chemical_Nomenclature : 1-[3-(3-bromopropoxy)-4-chloro-1-oxoisochromen-7-yl]-3-phenylurea
Canonical SMILES : C1=CC=C(C=C1)NC(=O)NC2=CC3=C(C=C2)C(=C(OC3=O)OCCCBr)Cl
InChI : InChI=1S\/C19H16BrClN2O4\/c20-9-4-10-26-18-16(21)14-8-7-13(11-15(14)17(24)27-18)23-19(25)22-12-5-2-1-3-6-12\/h1-3,5-8,11H,4,9-10H2,(H2,22,23,25)
InChIKey : NOUCBQBZTFYQQV-UHFFFAOYSA-N
Other name(s) : CHEMBL153671,SCHEMBL7320533,BDBM50227425,3-(3-Bromopropoxy)-4-chloro-7-(3-phenylureido)isocoumarin
MW : 451.7
Formula : C19H16BrClN2O4
CAS_number :
PubChem : 10433984
UniChem : NOUCBQBZTFYQQV-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families :
Stucture :
Protein :
References (1)
| Title : Identification of covalent inhibitors that disrupt M. tuberculosis growth by targeting multiple serine hydrolases involved in lipid metabolism - Babin_2022_Cell.Chem.Biol_29_897 |
| Author(s) : Babin BM , Keller LJ , Pinto Y , Li VL , Eneim AS , Vance SE , Terrell SM , Bhatt AS , Long JZ , Bogyo M |
| Ref : Cell Chemical Biology , 29 :897 , 2022 |
| Abstract : Babin_2022_Cell.Chem.Biol_29_897 |
| ESTHER : Babin_2022_Cell.Chem.Biol_29_897 |
| PubMedSearch : Babin_2022_Cell.Chem.Biol_29_897 |
| PubMedID: 34599874 |