MIDA-ester-4j
Specific inhibitor of ABHD3. MIDA boronate ester. 6-methyl-1,3,6,2-dioxazaborocane-4,8-dione (N-methyliminodiacetic acid) displays nanomolar in vitro and in situ IC50 values and fully inhibits ABHD3 activity in human cells could be nanomolar rang inhibitor
General
Type : MIDA boronate,Boron compound
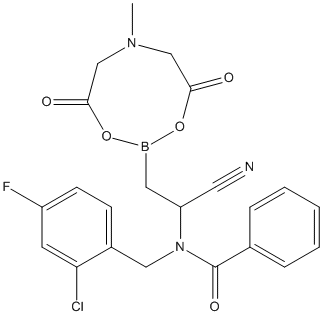
Chemical_Nomenclature : (2-(N-(2-chloro-4-fluorobenzyl)benzamido)-2-cyanoethyl) acid 6-methyl-1,3,6,2-dioxazaborocane-4,8-dione ester
Canonical SMILES : C1=C(C(=CC=C1F)CN(C(=O)C2=CC=CC=C2)C(C#N)CB3OC(CN(CC(O3)=O)C)=O)Cl
InChI : InChI=1S\/C22H20BClFN3O5\/c1-27-13-20(29)32-23(33-21(30)14-27)10-18(11-26)28(22(31)15-5-3-2-4-6-15)12-16-7-8-17(25)9-19(16)24\/h2-9,18H,10,12-14H2,1H3
InChIKey : GIXDQHPLLJDVGG-UHFFFAOYSA-N
Other name(s) : MIDA (2-(N-(2-chloro-4-fluorobenzyl)benzamido)-2-cyanoethyl)boronate,(2-(N-(2-chloro-4-fluorobenzyl)benzamido)-2-cyanoethyl)boronate MIDA ester
MW : 472.12
Formula : C22H21BClFN3O5
CAS_number :
PubChem :
UniChem : GIXDQHPLLJDVGG-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : MIDA-ester-4j ligand of proteins in family: abh_upf0017
Stucture :
Protein : human-ABHD3 || mouse-abhd3
References (1)
| Title : Multicomponent mapping of boron chemotypes furnishes selective enzyme inhibitors - Tan_2017_Nat.Commun_8_1760 |
| Author(s) : Tan J , Cognetta AB, 3rd , Diaz DB , Lum KM , Adachi S , Kundu S , Cravatt BF , Yudin AK |
| Ref : Nat Commun , 8 :1760 , 2017 |
| Abstract : Tan_2017_Nat.Commun_8_1760 |
| ESTHER : Tan_2017_Nat.Commun_8_1760 |
| PubMedSearch : Tan_2017_Nat.Commun_8_1760 |
| PubMedID: 29170371 |
| Gene_locus related to this paper: human-ABHD3 , mouse-abhd3 |