Malaoxon
Results from the metabolism in vivo of Malathion( oxygen replaces the sulfur atom)
General
Type : Insecticide,Organophosphate,Sulfur Compound,Organothiophosphate
Chemical_Nomenclature : diethyl 2-dimethoxyphosphorylsulfanylbutanedioate
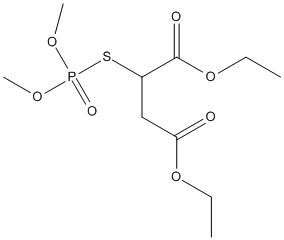
Canonical SMILES : CCOC(=O)CC(C(=O)OCC)SP(=O)(OC)OC
InChI : InChI=1S\/C10H19O7PS\/c1-5-16-9(11)7-8(10(12)17-6-2)19-18(13,14-3)15-4\/h8H,5-7H2,1-4H3
InChIKey : WSORODGWGUUOBO-UHFFFAOYSA-N
Other name(s) : diethyl((dimethoxyphosphinyl)thio)butanedioate,O,O-dimethyl-S-1,2-bis(ethoxycarbonyl)ethyl phosphorothioate,Malathion-o-analog
MW : 1634-78-2
Formula : C10H19O7PS
CAS_number : 314.29
PubChem : 15415
UniChem : WSORODGWGUUOBO-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Malaoxon
Target
References (5)
| Title : Inhibition of carboxylesterases in SH-SY5Y human and NB41A3 mouse neuroblastoma cells by organophosphorus esters - Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| Author(s) : Ehrich M , Correll L |
| Ref : J Toxicol Environ Health , 53 :385 , 1998 |
| Abstract : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| ESTHER : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedSearch : Ehrich_1998_J.Toxicol.Environ.Health_53_385 |
| PubMedID: 9515941 |
| Title : Rat brain acetylcholinesterase activity: developmental profile and maturational sensitivity to carbamate and organophosphorus inhibitors - Mortensen_1998_Toxicology_125_13 |
| Author(s) : Mortensen SR , Hooper MJ , Padilla S |
| Ref : Toxicology , 125 :13 , 1998 |
| Abstract : Mortensen_1998_Toxicology_125_13 |
| ESTHER : Mortensen_1998_Toxicology_125_13 |
| PubMedSearch : Mortensen_1998_Toxicology_125_13 |
| PubMedID: 9585096 |
| Title : Inhibition of various cholinesterases with the enantiomers of malaoxon - Rodriguez_1997_Bull.Environ.Contam.Toxicol_58_171 |
| Author(s) : Rodriguez OP , Muth GW , Berkman CE , Kim K , Thompson CM |
| Ref : Bulletin of Environmental Contamination & Toxicology , 58 :171 , 1997 |
| Abstract : Rodriguez_1997_Bull.Environ.Contam.Toxicol_58_171 |
| ESTHER : Rodriguez_1997_Bull.Environ.Contam.Toxicol_58_171 |
| PubMedSearch : Rodriguez_1997_Bull.Environ.Contam.Toxicol_58_171 |
| PubMedID: 8975790 |
| Title : Interaction of acetylcholinesterase with the enantiomers of malaoxon and isomalathion - Berkman_1993_Chem.Res.Toxicol_6_724 |
| Author(s) : Berkman CE , Quinn DA , Thompson CM |
| Ref : Chemical Research in Toxicology , 6 :724 , 1993 |
| Abstract : Berkman_1993_Chem.Res.Toxicol_6_724 |
| ESTHER : Berkman_1993_Chem.Res.Toxicol_6_724 |
| PubMedSearch : Berkman_1993_Chem.Res.Toxicol_6_724 |
| PubMedID: 8292752 |
| Title : Synthesis, absolute configuration, and analysis of malathion, malaoxon, and isomalathion enantiomers [published erratum appears in Chem Res Toxicol 1994 Mar-Apr\;7(2):275] - Berkman_1993_Chem.Res.Toxicol_6_718 |
| Author(s) : Berkman CE , Thompson CM , Perrin SR |
| Ref : Chemical Research in Toxicology , 6 :718 , 1993 |
| Abstract : Berkman_1993_Chem.Res.Toxicol_6_718 |
| ESTHER : Berkman_1993_Chem.Res.Toxicol_6_718 |
| PubMedSearch : Berkman_1993_Chem.Res.Toxicol_6_718 |
| PubMedID: 8292751 |