Memoquin
acetylcholinesterase and beta-secretase-1 inhibitor, and possesses anti-amyloid and anti-oxidant properties
General
Type : Multitarget,BACE1-inhibitor,Antioxidant,Alkyl linked bis-ligand
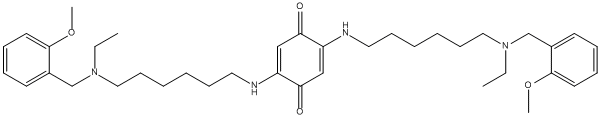
Chemical_Nomenclature : 2,5-bis[6-[ethyl-[(2-methoxyphenyl)methyl]amino]hexylamino]cyclohexa-2,5-diene-1,4-dione
Canonical SMILES : CCN(CCCCCCNC1=CC(=O)C(=CC1=O)NCCCCCCN(CC)CC2=CC=CC=C2OC)CC3=CC=CC=C3OC
InChI : InChI=1S\/C38H56N4O4\/c1-5-41(29-31-19-11-13-21-37(31)45-3)25-17-9-7-15-23-39-33-27-36(44)34(28-35(33)43)40-24-16-8-10-18-26-42(6-2)30-32-20-12-14-22-38(32)46-4\/h11-14,19-22,27-28,39-40H,5-10,15-18,23-26,29-30H2,1-4H3
InChIKey : VPXVPJQOPRBXPO-UHFFFAOYSA-N
Other name(s) : D01KVT,SCHEMBL4365745,ZINC101086292,KB-274293
MW : 632.89
Formula : C38H56N4O4
CAS_number : 616885-87-1
PubChem : 9917279
UniChem : VPXVPJQOPRBXPO-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (5)
| Title : Design, synthesis and evaluation of novel ferulic acid-memoquin hybrids as potential multifunctional agents for the treatment of Alzheimer's disease - Pan_2016_Bioorg.Med.Chem.Lett_26_2539 |
| Author(s) : Pan W , Hu K , Bai P , Yu L , Ma Q , Li T , Zhang X , Chen C , Peng K , Liu W , Sang Z |
| Ref : Bioorganic & Medicinal Chemistry Lett , 26 :2539 , 2016 |
| Abstract : Pan_2016_Bioorg.Med.Chem.Lett_26_2539 |
| ESTHER : Pan_2016_Bioorg.Med.Chem.Lett_26_2539 |
| PubMedSearch : Pan_2016_Bioorg.Med.Chem.Lett_26_2539 |
| PubMedID: 27072909 |
| Title : Pharmacological characterization of memoquin, a multi-target compound for the treatment of Alzheimer's disease - Capurro_2013_PLoS.One_8_e56870 |
| Author(s) : Capurro V , Busquet P , Lopes JP , Bertorelli R , Tarozzo G , Bolognesi ML , Piomelli D , Reggiani A , Cavalli A |
| Ref : PLoS ONE , 8 :e56870 , 2013 |
| Abstract : Capurro_2013_PLoS.One_8_e56870 |
| ESTHER : Capurro_2013_PLoS.One_8_e56870 |
| PubMedSearch : Capurro_2013_PLoS.One_8_e56870 |
| PubMedID: 23441223 |
| Title : Structure-activity relationships of memoquin: Influence of the chain chirality in the multi-target mechanism of action - Bolognesi_2009_Bioorg.Med.Chem.Lett_19_4312 |
| Author(s) : Bolognesi ML , Bartolini M , Rosini M , Andrisano V , Melchiorre C |
| Ref : Bioorganic & Medicinal Chemistry Lett , 19 :4312 , 2009 |
| Abstract : Bolognesi_2009_Bioorg.Med.Chem.Lett_19_4312 |
| ESTHER : Bolognesi_2009_Bioorg.Med.Chem.Lett_19_4312 |
| PubMedSearch : Bolognesi_2009_Bioorg.Med.Chem.Lett_19_4312 |
| PubMedID: 19505825 |
| Title : MTDL design strategy in the context of Alzheimer's disease: from lipocrine to memoquin and beyond - Bolognesi_2009_Curr.Pharm.Des_15_601 |
| Author(s) : Bolognesi ML , Rosini M , Andrisano V , Bartolini M , Minarini A , Tumiatti V , Melchiorre C |
| Ref : Curr Pharm Des , 15 :601 , 2009 |
| Abstract : Bolognesi_2009_Curr.Pharm.Des_15_601 |
| ESTHER : Bolognesi_2009_Curr.Pharm.Des_15_601 |
| PubMedSearch : Bolognesi_2009_Curr.Pharm.Des_15_601 |
| PubMedID: 19199985 |
| Title : Toward a rational design of multitarget-directed antioxidants: merging memoquin and lipoic acid molecular frameworks - Bolognesi_2009_J.Med.Chem_52_7883 |
| Author(s) : Bolognesi ML , Cavalli A , Bergamini C , Fato R , Lenaz G , Rosini M , Bartolini M , Andrisano V , Melchiorre C |
| Ref : Journal of Medicinal Chemistry , 52 :7883 , 2009 |
| Abstract : Bolognesi_2009_J.Med.Chem_52_7883 |
| ESTHER : Bolognesi_2009_J.Med.Chem_52_7883 |
| PubMedSearch : Bolognesi_2009_J.Med.Chem_52_7883 |
| PubMedID: 19813747 |