Methiocarb
General
Type : Carbamate,Insecticide,Sulfur Compound
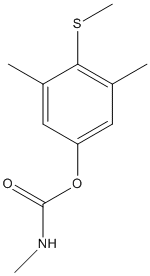
Chemical_Nomenclature : (3,5-dimethyl-4-methylsulfanylphenyl) N-methylcarbamate
Canonical SMILES : CC1=CC(=CC(=C1SC)C)OC(=O)NC
InChI : InChI=1S\/C11H15NO2S\/c1-7-5-9(14-11(13)12-3)6-8(2)10(7)15-4\/h5-6H,1-4H3,(H,12,13)
InChIKey : YFBPRJGDJKVWAH-UHFFFAOYSA-N
Other name(s) : Mercaptodimethur,Metmercapturon,Mesurol,Methiocarbe
MW : 225.30
Formula : C11H15NO2S
CAS_number : 2032-65-7
PubChem : 16248
UniChem : YFBPRJGDJKVWAH-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Methiocarb
Target
Families : Methiocarb ligand of proteins in family: ACHE || BCHE || Carb_B_Arthropoda
Stucture :
Protein :
References (7)
| Title : Acute effects of methiocarb on oxidative damage and the protective effects of vitamin E and taurine in the liver and kidney of Wistar rats - Ozden_2013_Toxicol.Ind.Health_29_60 |
| Author(s) : Ozden S , Catalgol B , Gezginci-Oktayoglu S , Karatug A , Bolkent S , Alpertunga B |
| Ref : Toxicol Ind Health , 29 :60 , 2013 |
| Abstract : Ozden_2013_Toxicol.Ind.Health_29_60 |
| ESTHER : Ozden_2013_Toxicol.Ind.Health_29_60 |
| PubMedSearch : Ozden_2013_Toxicol.Ind.Health_29_60 |
| PubMedID: 22623520 |
| Title : Esterase inhibition by synergists in the western flower thrips Frankliniella occidentalis - Lopez-Soler_2011_Pest.Manag.Sci_67_1549 |
| Author(s) : Lopez-Soler N , Cervera A , Quinto V , Abellan J , Bielza P , Martinez-Pardo R , Garcera MD |
| Ref : Pest Manag Sci , 67 :1549 , 2011 |
| Abstract : Lopez-Soler_2011_Pest.Manag.Sci_67_1549 |
| ESTHER : Lopez-Soler_2011_Pest.Manag.Sci_67_1549 |
| PubMedSearch : Lopez-Soler_2011_Pest.Manag.Sci_67_1549 |
| PubMedID: 21656898 |
| Title : Time course of cholinesterase inhibition in adult rats treated acutely with carbaryl, carbofuran, formetanate, methomyl, methiocarb, oxamyl or propoxur - Padilla_2007_Toxicol.Appl.Pharmacol_219_202 |
| Author(s) : Padilla S , Marshall RS , Hunter DL , Lowit A |
| Ref : Toxicol Appl Pharmacol , 219 :202 , 2007 |
| Abstract : Padilla_2007_Toxicol.Appl.Pharmacol_219_202 |
| ESTHER : Padilla_2007_Toxicol.Appl.Pharmacol_219_202 |
| PubMedSearch : Padilla_2007_Toxicol.Appl.Pharmacol_219_202 |
| PubMedID: 17197007 |
| Title : Relationship between esterase activity and acrinathrin and methiocarb resistance in field populations of western flower thrips, Frankliniella occidentalis - Mayma_2006_Pest.Manag.Sci_62_1129 |
| Author(s) : Mayma AC , Cervera A , Dolores Garcera M , Bielza P , Martinez-Pardo R |
| Ref : Pest Manag Sci , 62 :1129 , 2006 |
| Abstract : Mayma_2006_Pest.Manag.Sci_62_1129 |
| ESTHER : Mayma_2006_Pest.Manag.Sci_62_1129 |
| PubMedSearch : Mayma_2006_Pest.Manag.Sci_62_1129 |
| PubMedID: |
| Title : Mechanisms associated with methiocarb resistance in Frankliniella occidentalis (Thysanoptera: Thripidae) - Jensen_2000_J.Econ.Entomol_93_464 |
| Author(s) : Jensen SE |
| Ref : J Econ Entomol , 93 :464 , 2000 |
| Abstract : Jensen_2000_J.Econ.Entomol_93_464 |
| ESTHER : Jensen_2000_J.Econ.Entomol_93_464 |
| PubMedSearch : Jensen_2000_J.Econ.Entomol_93_464 |
| PubMedID: 10826201 |
| Title : Acetylcholinesterase Activity Associated with Methiocarb Resistance in a Strain of Western Flower Thrips, Frankliniella occidentalis (Pergande) - Jensen_1998_Pestic.Biochem.Physiol_61_191 |
| Author(s) : Jensen SE |
| Ref : Pesticide Biochemistry and Physiology , 61 :191 , 1998 |
| Abstract : Jensen_1998_Pestic.Biochem.Physiol_61_191 |
| ESTHER : Jensen_1998_Pestic.Biochem.Physiol_61_191 |
| PubMedSearch : Jensen_1998_Pestic.Biochem.Physiol_61_191 |
| PubMedID: |
| Title : Stereoselective sulfoxidation of the pesticide methiocarb by flavin-containing monooxygenase and cytochrome P450-dependent monooxygenases of rat liver microsomes. Anticholinesterase activity of the two sulfoxide enantiomers - Buronfosse_1995_J.Biochem.Toxicol_10_179 |
| Author(s) : Buronfosse T , Moroni P , Benoit E , Riviere JL |
| Ref : Journal of Biochemical Toxicology , 10 :179 , 1995 |
| Abstract : Buronfosse_1995_J.Biochem.Toxicol_10_179 |
| ESTHER : Buronfosse_1995_J.Biochem.Toxicol_10_179 |
| PubMedSearch : Buronfosse_1995_J.Biochem.Toxicol_10_179 |
| PubMedID: 8568832 |