Methylprednisolone
Methylprednisolone is a synthetic corticosteroid with anti-inflammatory and immunomodulating properties. Methylprednisolone binds to and activates specific nuclear receptors, resulting in altered gene expression and inhibition of proinflammatory cytokine production. This agent also decreases the number of circulating lymphocytes, induces cell differentiation, and stimulates apoptosis in sensitive tumor cell populations.
General
Type : Drug,Steroid,Phenanthrene,Not A\/B H target
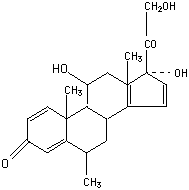
Chemical_Nomenclature : (6S,8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-6,10,13-trimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one
Canonical SMILES : CC1CC2C3CCC(C3(CC(C2C4(C1=CC(=O)C=C4)C)O)C)(C(=O)CO)O
InChI : InChI=1S\/C22H30O5\/c1-12-8-14-15-5-7-22(27,18(26)11-23)21(15,3)10-17(25)19(14)20(2)6-4-13(24)9-16(12)20\/h4,6,9,12,14-15,17,19,23,25,27H,5,7-8,10-11H2,1-3H3\/t12-,14-,15-,17-,19+,20-,21-,22-\/m0\/s1
InChIKey : VHRSUDSXCMQTMA-PJHHCJLFSA-N
Other name(s) : Medrol,Medrone,Urbason,Medrate,Metastab,Metrisone,Promacortine,Suprametil,Solumedrol,Depo-Medrol,Methylone,Solu-Medrol
MW : 374.48
Formula : C22H30O5
CAS_number : 83-43-2
PubChem : 6741
UniChem : VHRSUDSXCMQTMA-PJHHCJLFSA-N
IUPHAR :
Wikipedia : Methylprednisolone
Target
References (2)
| Title : Amplification of neuromuscular transmission by methylprednisolone involves activation of presynaptic facilitatory adenosine A receptors and redistribution of synaptic vesicles - Oliveira_2014_Neuropharmacol_89C_64 |
| Author(s) : Oliveira L , Costa AC , Noronha-Matos JB , Silva I , Cavalcante WL , Timoteo MA , Corrado AP , Dal Belo CA , Ambiel CR , Alves-do-Prado W , Correia-de-Sa P |
| Ref : Neuropharmacology , 89C :64 , 2014 |
| Abstract : Oliveira_2014_Neuropharmacol_89C_64 |
| ESTHER : Oliveira_2014_Neuropharmacol_89C_64 |
| PubMedSearch : Oliveira_2014_Neuropharmacol_89C_64 |
| PubMedID: 25220030 |
| Title : Hydrolysis of methylprednisolone acetate by human serum cholinesterase - |
| Author(s) : Myers C , Lockridge O , La Du BN |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 10 :279 , 1982 |
| PubMedID: 6125364 |