Nicotine
Prototype nicotinic agonist; naturally occuring isomer || Nicotine is a plant alkaloid, found in the tobacco plant, and addictive central nervous system (CNS) stimulant that causes either ganglionic stimulation in low doses or ganglionic blockage in high doses. Nicotine acts as an agonist at the nicotinic cholinergic receptors in the autonomic ganglia, at neuromuscular junctions, and in the adrenal medulla and the brain. Nicotine's CNS-stimulating activities may be mediated through the release of several neurotransmitters, including acetylcholine, beta-endorphin, dopamine, norepinephrine, serotonin, and ACTH. As a result, peripheral vasoconstriction, tachycardia, and elevated blood pressure may be observed with nicotine intake. This agent may also stimulate the chemoreceptor trigger zone, thereby inducing nausea and vomiting.
General
Type : Derivative of Nicotine,Pyrrolidine,Natural,Alkaloid,Drug,Nicotinic agonist,Not A\/B H target
Chemical_Nomenclature : [-]-1-Methyl-2-[3-pyridyl]pyrrolidine || 3-[(2S)-1-methylpyrrolidin-2-yl]pyridine
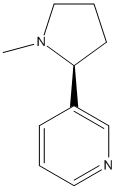
Canonical SMILES : CN1CCCC1C2=CN=CC=C2
InChI : InChI=1S\/C10H14N2\/c1-12-7-3-5-10(12)9-4-2-6-11-8-9\/h2,4,6,8,10H,3,5,7H2,1H3\/t10-\/m0\/s1 || InChI=1S\/C10H14N2\/c1-12-7-3-5-10(12)9-4-2-6-11-8-9\/h2,4,6,8,10H,3,5,7H2,1H3
InChIKey : SNICXCGAKADSCV-JTQLQIEISA-N || SNICXCGAKADSCV-UHFFFAOYSA-N
Other name(s) : L-Nicotine,(-)-Nicotine,(S)-Nicotine,CHEBI:17688,SCHEMBL20192,ZINC391812,DB00184,SCHEMBL11976729,CHEBI:18723,CHEBI:138000
MW : 162.2
Formula : C10H14N2
CAS_number : 54-11-5 || 22083-74-5
UniChem : SNICXCGAKADSCV-JTQLQIEISA-N || SNICXCGAKADSCV-UHFFFAOYSA-N
IUPHAR : 2585
Wikipedia : Nicotine
Target
Families : Nicotine ligand of proteins in family: ACHE || BCHE || Pectinacetylesterase-Notum
Stucture : 7BNE Human Notum complexed with Nicotine
Protein : human-NOTUM
References (1)
| Title : Structure-activity relationship of reversible cholinesterase inhibitors including paraquat - Seto_1988_Arch.Toxicol_62_37 |
| Author(s) : Seto Y , Shinohara T |
| Ref : Archives of Toxicology , 62 :37 , 1988 |
| Abstract : Seto_1988_Arch.Toxicol_62_37 |
| ESTHER : Seto_1988_Arch.Toxicol_62_37 |
| PubMedSearch : Seto_1988_Arch.Toxicol_62_37 |
| PubMedID: 3190453 |