Palmitate
Palmitic acid is a common saturated fatty acid with a 16-carbon backbone found in fats and waxes including olive oil, palm oil, as well as in butter, cheese, milk and meat. Product of hydrolysis of Palmitate esters
General
Type : Natural,Fatty acid,Hexadecanoate,Palmitate
Chemical_Nomenclature : hexadecanoic acid
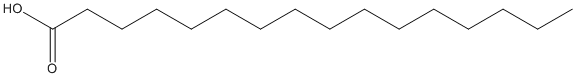
Canonical SMILES : CCCCCCCCCCCCCCCC(=O)O
InChI : InChI=1S\/C16H32O2\/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(17)18\/h2-15H2,1H3,(H,17,18)
InChIKey : IPCSVZSSVZVIGE-UHFFFAOYSA-N
Other name(s) : Palmitic acid,Hexadecanoic acid,Cetylic acid,C16 fatty acid,C16:0,N-Hexadecanoic acid
MW : 256.43
Formula : C16H32O2
CAS_number : 57-10-3
PubChem : 985
UniChem : IPCSVZSSVZVIGE-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Palmitate ligand of proteins in family: Homoserine_transacetylase || Carb_B_Chordata || Palmitoyl-protein_thioesterase || Lipase_3 || Tiancimycin-TnmK-Tripeptidase-HIP || PGAP1
Stucture :
Protein : bacan-BA4983 || human-CES1 || bovin-ppt || humla-1lipa || myctu-ym24 || chatd-g0s652
References (3)
| Title : The Crystal Structures of Thermomyces (Humicola) Lanuginosa Lipase in Complex with Enzymatic Reactants - McPherson_2020_Curr.Enzym.Inhib_16_ |
| Author(s) : McPherson A , Larson SB , Kalasky A |
| Ref : Curr Enzym Inhib , 16 : , 2020 |
| Abstract : McPherson_2020_Curr.Enzym.Inhib_16_ |
| ESTHER : McPherson_2020_Curr.Enzym.Inhib_16_ |
| PubMedSearch : McPherson_2020_Curr.Enzym.Inhib_16_ |
| PubMedID: |
| Gene_locus related to this paper: humla-1lipa |
| Title : Multisite promiscuity in the processing of endogenous substrates by human carboxylesterase 1 - Bencharit_2006_J.Mol.Biol_363_201 |
| Author(s) : Bencharit S , Edwards CC , Morton CL , Howard-Williams EL , Kuhn P , Potter PM , Redinbo MR |
| Ref : Journal of Molecular Biology , 363 :201 , 2006 |
| Abstract : Bencharit_2006_J.Mol.Biol_363_201 |
| ESTHER : Bencharit_2006_J.Mol.Biol_363_201 |
| PubMedSearch : Bencharit_2006_J.Mol.Biol_363_201 |
| PubMedID: 16962139 |
| Gene_locus related to this paper: human-CES1 |
| Title : The crystal structure of palmitoyl protein thioesterase 1 and the molecular basis of infantile neuronal ceroid lipofuscinosis - Bellizzi_2000_Proc.Natl.Acad.Sci.U.S.A_97_4573 |
| Author(s) : Bellizzi JJ, 3rd , Widom J , Kemp C , Lu JY , Das AK , Hofmann SL , Clardy J |
| Ref : Proc Natl Acad Sci U S A , 97 :4573 , 2000 |
| Abstract : Bellizzi_2000_Proc.Natl.Acad.Sci.U.S.A_97_4573 |
| ESTHER : Bellizzi_2000_Proc.Natl.Acad.Sci.U.S.A_97_4573 |
| PubMedSearch : Bellizzi_2000_Proc.Natl.Acad.Sci.U.S.A_97_4573 |
| PubMedID: 10781062 |
| Gene_locus related to this paper: bovin-ppt , human-PPT1 |