Panclicin-D
General
Type : Oxooxetan,Natural
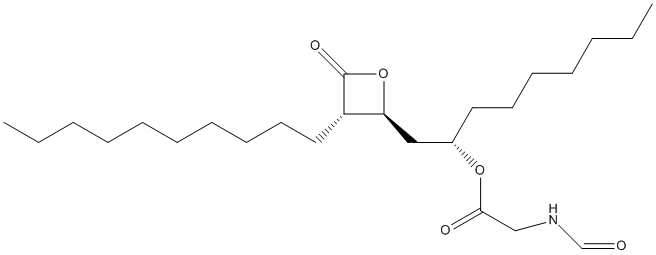
Chemical_Nomenclature : [(2S)-1-[(2S,3S)-3-decyl-4-oxooxetan-2-yl]nonan-2-yl] 2-formamidoacetate
Canonical SMILES : CCCCCCCCCCC1C(OC1=O)CC(CCCCCCC)OC(=O)CNC=O
InChI : InChI=1S\/C25H45NO5\/c1-3-5-7-9-10-11-13-15-17-22-23(31-25(22)29)18-21(16-14-12-8-6-4-2)30-24(28)19-26-20-27\/h20-23H,3-19H2,1-2H3,(H,26,27)\/t21-,22-,23-\/m0\/s1
InChIKey : RQFZMIXBLJEUGS-VABKMULXSA-N
Other name(s) : Panclicin D,SCHEMBL713881
MW : 439.62
Formula : C25H45NO5
CAS_number : 160669-44-3
PubChem : 9889418
UniChem : RQFZMIXBLJEUGS-VABKMULXSA-N
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Panclicins, novel pancreatic lipase inhibitors. II. Structural elucidation - Yoshinari_1994_J.Antibiot.(Tokyo)_47_1376 |
| Author(s) : Yoshinari K , Aoki M , Ohtsuka T , Nakayama N , Itezono Y , Mutoh M , Watanabe J , Yokose K |
| Ref : J Antibiot (Tokyo) , 47 :1376 , 1994 |
| Abstract : Yoshinari_1994_J.Antibiot.(Tokyo)_47_1376 |
| ESTHER : Yoshinari_1994_J.Antibiot.(Tokyo)_47_1376 |
| PubMedSearch : Yoshinari_1994_J.Antibiot.(Tokyo)_47_1376 |
| PubMedID: 7844032 |