Permethrin
Not an inhibitor. See Permethrin as substrate Permethrin || The target is prolonging sodium channel activation. Only few Pyrethroids interact with carboxylesterases. Pyrethrins are naturally-occurring compounds with insecticidal properties that are found in pyrethrum extract from certain chrysanthemum flowers. The pyrethrins are often used in household insecticides and products to control insects on pets or livestock. Pyrethroids are manufactured chemicals that are very similar in structure to the pyrethrins, but are often more toxic to insects as well as to mammals, and last longer in the environment than the pyrethrins.
General
Type : Pyrethroid,Insecticide,Not A\/B H target,Phenoxyphenyl,Cyclopropyl
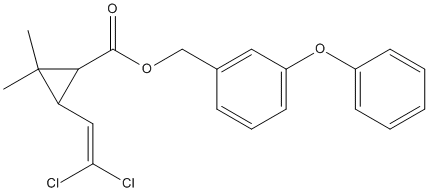
Chemical_Nomenclature : (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate
Canonical SMILES : CC1(C(C1C(=O)OCC2=CC(=CC=C2)OC3=CC=CC=C3)C=C(Cl)Cl)C
InChI : InChI=1S\/C21H20Cl2O3\/c1-21(2)17(12-18(22)23)19(21)20(24)25-13-14-7-6-10-16(11-14)26-15-8-4-3-5-9-15\/h3-12,17,19H,13H2,1-2H3
InChIKey : RLLPVAHGXHCWKJ-UHFFFAOYSA-N
Other name(s) : Transpermethrin,Ambush,Pounce,1RS,cis-Permethrin,Permethrine,Ambushfog,Chinetrin,Efmethrin,Imperator,Indothrin,Outflank,Permasect,Perthrine,Ectiban,FMC 33297,BW-21-Z,Eksmin,Kafil,Pramex,Talcord,Nix,Dragnet
MW : 391.29
Formula : C21H20Cl2O3
CAS_number : 52645-53-1
PubChem : 40326
UniChem : RLLPVAHGXHCWKJ-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Permethrin
Target
Families :
Stucture :
Protein :
References (11)
| Title : Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin - Yang_2009_Biochem.Pharmacol_77_238 |
| Author(s) : Yang D , Pearce RE , Wang X , Gaedigk R , Wan YJ , Yan B |
| Ref : Biochemical Pharmacology , 77 :238 , 2009 |
| Abstract : Yang_2009_Biochem.Pharmacol_77_238 |
| ESTHER : Yang_2009_Biochem.Pharmacol_77_238 |
| PubMedSearch : Yang_2009_Biochem.Pharmacol_77_238 |
| PubMedID: 18983829 |
| Gene_locus related to this paper: human-CES1 , human-CES2 |
| Title : Beta-cypermethrin resistance associated with high carboxylesterase activities in a strain of house fly, Musca domestica (Diptera: Muscidae) - Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| Author(s) : Zhang L , Gao X , Liang P |
| Ref : Pesticide Biochemistry and Physiology , 89 :65 , 2007 |
| Abstract : Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| ESTHER : Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| PubMedSearch : Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| PubMedID: |
| Title : Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver and serum carboxylesterases - Crow_2007_Toxicol.Appl.Pharmacol_221_1 |
| Author(s) : Crow JA , Borazjani A , Potter PM , Ross MK |
| Ref : Toxicol Appl Pharmacol , 221 :1 , 2007 |
| Abstract : Crow_2007_Toxicol.Appl.Pharmacol_221_1 |
| ESTHER : Crow_2007_Toxicol.Appl.Pharmacol_221_1 |
| PubMedSearch : Crow_2007_Toxicol.Appl.Pharmacol_221_1 |
| PubMedID: 17442360 |
| Title : The in vitro metabolism of a pyrethroid insecticide, permethrin, and its hydrolysis products in rats - Nakamura_2007_Toxicology_235_176 |
| Author(s) : Nakamura Y , Sugihara K , Sone T , Isobe M , Ohta S , Kitamura S |
| Ref : Toxicology , 235 :176 , 2007 |
| Abstract : Nakamura_2007_Toxicology_235_176 |
| ESTHER : Nakamura_2007_Toxicology_235_176 |
| PubMedSearch : Nakamura_2007_Toxicology_235_176 |
| PubMedID: 17451859 |
| Title : Characterization of pyrethroid hydrolysis by the human liver carboxylesterases hCE-1 and hCE-2 - Nishi_2006_Arch.Biochem.Biophys_445_115 |
| Author(s) : Nishi K , Huang H , Kamita SG , Kim IH , Morisseau C , Hammock BD |
| Ref : Archives of Biochemistry & Biophysics , 445 :115 , 2006 |
| Abstract : Nishi_2006_Arch.Biochem.Biophys_445_115 |
| ESTHER : Nishi_2006_Arch.Biochem.Biophys_445_115 |
| PubMedSearch : Nishi_2006_Arch.Biochem.Biophys_445_115 |
| PubMedID: 16359636 |
| Gene_locus related to this paper: human-CES1 , human-CES2 |
| Title : Hydrolysis of pyrethroids by carboxylesterases from Lucilia cuprina and Drosophila melanogaster with active sites modified by in vitro mutagenesis - Heidari_2005_Insect.Biochem.Mol.Biol_35_597 |
| Author(s) : Heidari R , Devonshire AL , Campbell BE , Dorrian SJ , Oakeshott JG , Russell RJ |
| Ref : Insect Biochemistry & Molecular Biology , 35 :597 , 2005 |
| Abstract : Heidari_2005_Insect.Biochem.Mol.Biol_35_597 |
| ESTHER : Heidari_2005_Insect.Biochem.Mol.Biol_35_597 |
| PubMedSearch : Heidari_2005_Insect.Biochem.Mol.Biol_35_597 |
| PubMedID: 15857765 |
| Gene_locus related to this paper: drome-EST23aes07 , luccu-E3aest7 |
| Title : Identification, expression, and purification of a pyrethroid-hydrolyzing carboxylesterase from mouse liver microsomes - Stok_2004_J.Biol.Chem_279_29863 |
| Author(s) : Stok JE , Huang H , Jones PD , Wheelock CE , Morisseau C , Hammock BD |
| Ref : Journal of Biological Chemistry , 279 :29863 , 2004 |
| Abstract : Stok_2004_J.Biol.Chem_279_29863 |
| ESTHER : Stok_2004_J.Biol.Chem_279_29863 |
| PubMedSearch : Stok_2004_J.Biol.Chem_279_29863 |
| PubMedID: 15123619 |
| Gene_locus related to this paper: mouse-Ces2a , mouse-Ces2e |
| Title : Isolation and identification of an esterase from a Mexican strain of Boophilus microplus (Acari: Ixodidae) - Pruett_2002_J.Econ.Entomol_95_1001 |
| Author(s) : Pruett JH , Guerrero FD , Hernandez R |
| Ref : J Econ Entomol , 95 :1001 , 2002 |
| Abstract : Pruett_2002_J.Econ.Entomol_95_1001 |
| ESTHER : Pruett_2002_J.Econ.Entomol_95_1001 |
| PubMedSearch : Pruett_2002_J.Econ.Entomol_95_1001 |
| PubMedID: 12403427 |
| Title : Influence of permethrin, diazinon and ivermectin treatments on insecticide resistance in the horn fly (Diptera: Muscidae) - Byford_1999_Int.J.Parasitol_29_125 |
| Author(s) : Byford RL , Craig ME , DeRouen SM , Kimball MD , Morrison DG , Wyatt WE , Foil LD |
| Ref : International Journal for Parasitology , 29 :125 , 1999 |
| Abstract : Byford_1999_Int.J.Parasitol_29_125 |
| ESTHER : Byford_1999_Int.J.Parasitol_29_125 |
| PubMedSearch : Byford_1999_Int.J.Parasitol_29_125 |
| PubMedID: 10048825 |
| Title : Permethrin Carboxylesterase Functions as Nonspecific Sequestration Proteins in the Hemolymph of Colorado Potato Beetle, - Lee_1998_Pestic.Biochem.Physiol_62_51 |
| Author(s) : Lee SH , Clark JM |
| Ref : Pesticide Biochemistry and Physiology , 62 :51 , 1998 |
| Abstract : Lee_1998_Pestic.Biochem.Physiol_62_51 |
| ESTHER : Lee_1998_Pestic.Biochem.Physiol_62_51 |
| PubMedSearch : Lee_1998_Pestic.Biochem.Physiol_62_51 |
| PubMedID: |
| Title : Insecticide susceptibility in mosquitoes (Diptera: Culicidae) from French Polynesia - Failloux_1994_J.Med.Entomol_31_639 |
| Author(s) : Failloux AB , Ung A , Raymond M , Pasteur N |
| Ref : Journal of Medical Entomology , 31 :639 , 1994 |
| Abstract : Failloux_1994_J.Med.Entomol_31_639 |
| ESTHER : Failloux_1994_J.Med.Entomol_31_639 |
| PubMedSearch : Failloux_1994_J.Med.Entomol_31_639 |
| PubMedID: 7966164 |