Pyridophen-10
Inhibits AChE selectively over BChE, with a bimolecular rate constant similar to pyridostigmine. [11C]MDDP; N-[11C]Methyl-3-[[(dimethylamino)carbonyl]oxy]-2-(2',2'-diphenylpropionoxymethyl)pyridinium. Used as PET imaging of a novel potential heart acetylcholinesterase tracer by Wang et al.
General
Type : Muscarinic antagonist,Propionate,Carbamate,Pro-Drug,Multitarget,Not A\/B H target,PET probe
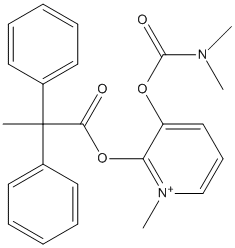
Chemical_Nomenclature : [3-(dimethylcarbamoyloxy)-1-methylpyridin-1-ium-2-yl]methyl 2,2-diphenylpropanoate
Canonical SMILES : CC(C1=CC=CC=C1)(C2=CC=CC=C2)C(=O)OCC3=C(C=CC=[N+]3C)OC(=O)N(C)C
InChI : InChI=1S\/C25H27N2O4\/c1-25(19-12-7-5-8-13-19,20-14-9-6-10-15-20)23(28)30-18-21-22(16-11-17-27(21)4)31-24(29)26(2)3\/h5-17H,18H2,1-4H3\/q+1
InChIKey : WHIMEOMPXHBKBV-UHFFFAOYSA-N
Other name(s) : CHEMBL443499,CHEMBL1185972,BDBM50109574,2-[(2,2-Diphenylpropionyloxy)methyl]-3-[(dimethylcarbamoyl)oxy]-1-methylpyridinium,3-Dimethylcarbamoyloxy-2-(2,2-diphenyl-propionyloxymethyl)-1-methyl-pyridinium \; methylsulfate
MW : 419.5
Formula : C25H27N2O4+
CAS_number :
PubChem : 10482128, 11734279, 10482127, 16040296
UniChem : WHIMEOMPXHBKBV-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Facile synthesis and PET imaging of a novel potential heart acetylcholinesterase tracer N-[11C]methyl-3-[[(dimethylamino)carbonyl]oxy]-2-(2',2'-diphenylpropionoxymethyl) pyridinium - Wang_2005_Bioorg.Med.Chem.Lett_15_4510 |
| Author(s) : Wang JQ , Miller MA , Mock BH , Lopshire JC , Groh WJ , Zipes DP , Hutchins GD , Zheng QH |
| Ref : Bioorganic & Medicinal Chemistry Lett , 15 :4510 , 2005 |
| Abstract : Wang_2005_Bioorg.Med.Chem.Lett_15_4510 |
| ESTHER : Wang_2005_Bioorg.Med.Chem.Lett_15_4510 |
| PubMedSearch : Wang_2005_Bioorg.Med.Chem.Lett_15_4510 |
| PubMedID: 16112863 |
| Title : Pyridophens: binary pyridostigmine-aprophen prodrugs with differential inhibition of acetylcholinesterase, butyrylcholinesterase, and muscarinic receptors - Leader_2002_J.Med.Chem_45_902 |
| Author(s) : Leader H , Wolfe AD , Chiang PK , Gordon RK |
| Ref : Journal of Medicinal Chemistry , 45 :902 , 2002 |
| Abstract : Leader_2002_J.Med.Chem_45_902 |
| ESTHER : Leader_2002_J.Med.Chem_45_902 |
| PubMedSearch : Leader_2002_J.Med.Chem_45_902 |
| PubMedID: 11831902 |